Expand the possibilities of drug delivery with innovative analytical solutions for lipid raw materials, lipid nanoparticles (LNPs) and other non-viral carriers. Move beyond boundaries in structural elucidation and monitoring of analytes to estabilish confidence in the quality and stablity of your products.
Lipid nanoparticles and non-viral carriers
Explore the latest and trending topics
-
Webinar
Better mRNA-LNPs: encapsulation efficiency, mRNA integrity and purity, lipid N-oxides and beyond
To ensure the safety and efficacy of LNP-based drugs, comprehensive characterization of these sophisticated molecules—the lipids and the genetic cargo, such as messenger RNA (mRNA)—is paramount. This webinar explores the analytical challenges of characterizing mRNA-LNPs and a range of potential solutions.
-
Webinar
Adducts and N-oxides: Understanding lipid nanoparticles (LNPs) for better mRNA-based drugs
Imagine taking control of your product quality and overcoming persistent challenges. Reclaim your time with a full solution for structural elucidation of complex lipids using EAD and automated data processing.
Overview
Forge a new path to better lipid nanoparticle (LNP)-based gene therapies and vaccines with solutions for lipid structural elucidation that reach unprecedented depth and quantitation accuracy, precision and sensitivity.
Turn ionizable lipid N-oxidation, double-bond saturations and other lipid impurity challenges into opportunities. Join the team to rewrite the rules of lipid analysis with workflows looking beyond the known.
Lipid structural elucidation
Workflow
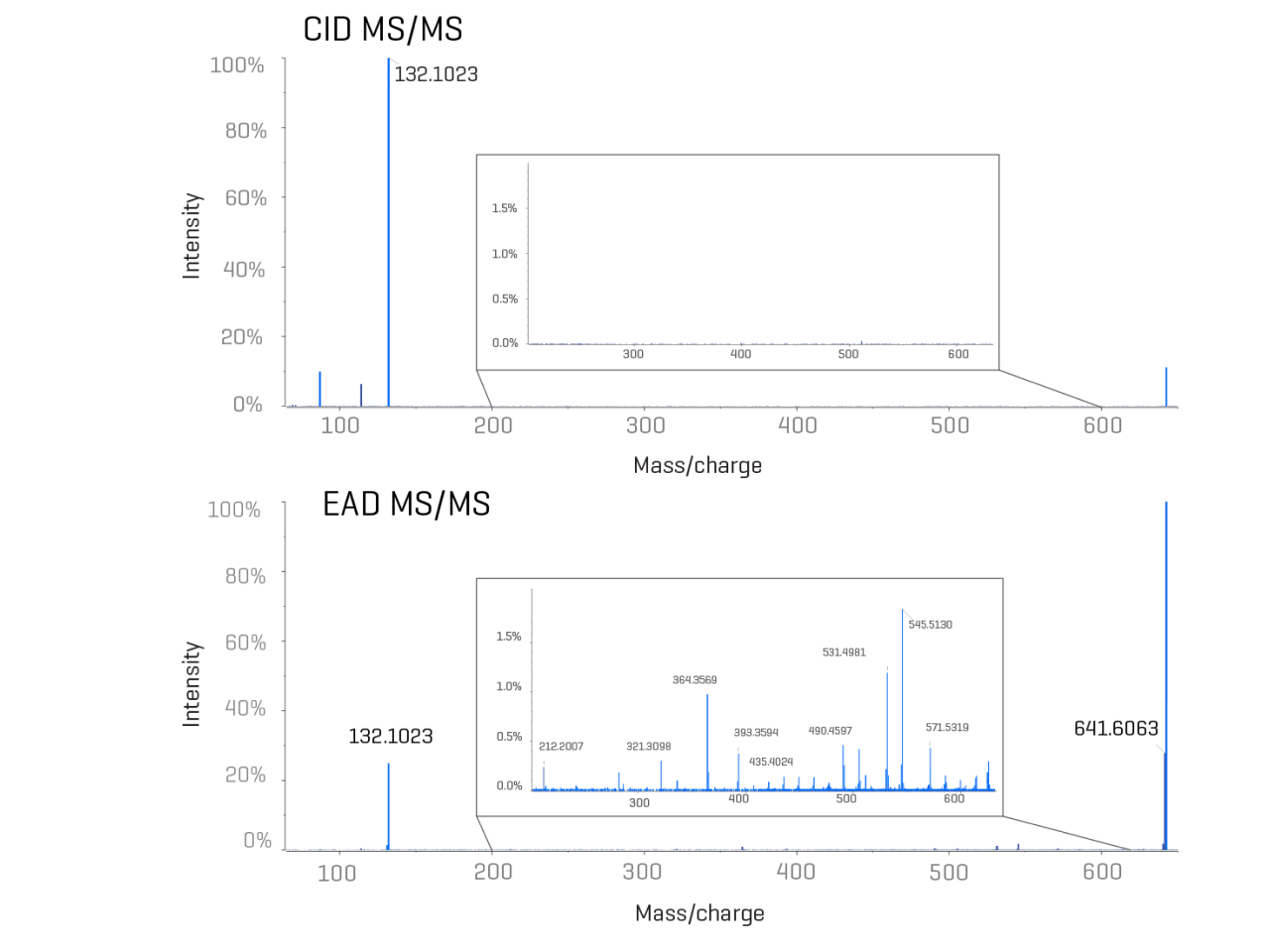
Take your journey further with the ability to identify and quantify the lipid impurities present at well below 0.1% relative intensity.
Workflows with novel fragmentation mechanisms overcome the limitations of traditional techniques, allowing full structural assessment of lipids and their impurities from raw materials and LNPs. Customizable, automated and data-driven solutions for lipid structural elucidation let you break free from the boundaries of drug formulation development.
- Fully understand the structures of your ionizable lipids and impurities
- Identify different oxygen incorporations, including N-oxides, saturated double bonds and more
- Reclaim your time with automatic processing of complex data
- Find relevant product excipients using state-of-the-art mass spectrometry solutions
- Benefit from simultaneous quantitation capabilities
Solution
Lipid structural elucidation
Suited for:
- In-depth structural elucidation
- Relative quantitation
Featured resources
-
Technical note
Characterization of the ionizable lipid MC3 and its impurities
Break down frontiers of structural characterization of lipids and their impurities to develop better drugs. Dedicated solutions help your team confidently advance the next generation of therapies and vaccines.
-
Webinar
Adducts and N-oxides: Understanding LNPs for better mRNA-based drugs
Take control of lipid impurities! Learn how to save your team's time and resources by leveraging full solutions that enable you to break through the boundaries around the structural identification of lipids and their impurities.
-
Technical note
Comparison of lots of ionizable lipid ALC-0315
Create pathways to know more about your non-viral carrier. Discover solutions that reliably identify process-related lipid impurities from raw material and LNPs.
-
Q&A blog
Adducts and N-oxides: Understanding lipid nanoparticles (LNPs) for better mRNA drugs
Hear from expert Adam Crowe (Cytiva) about lipid impurities, the associated risks and how to develop better LNP-based drugs.
Lipid impurity quantitation
Workflow
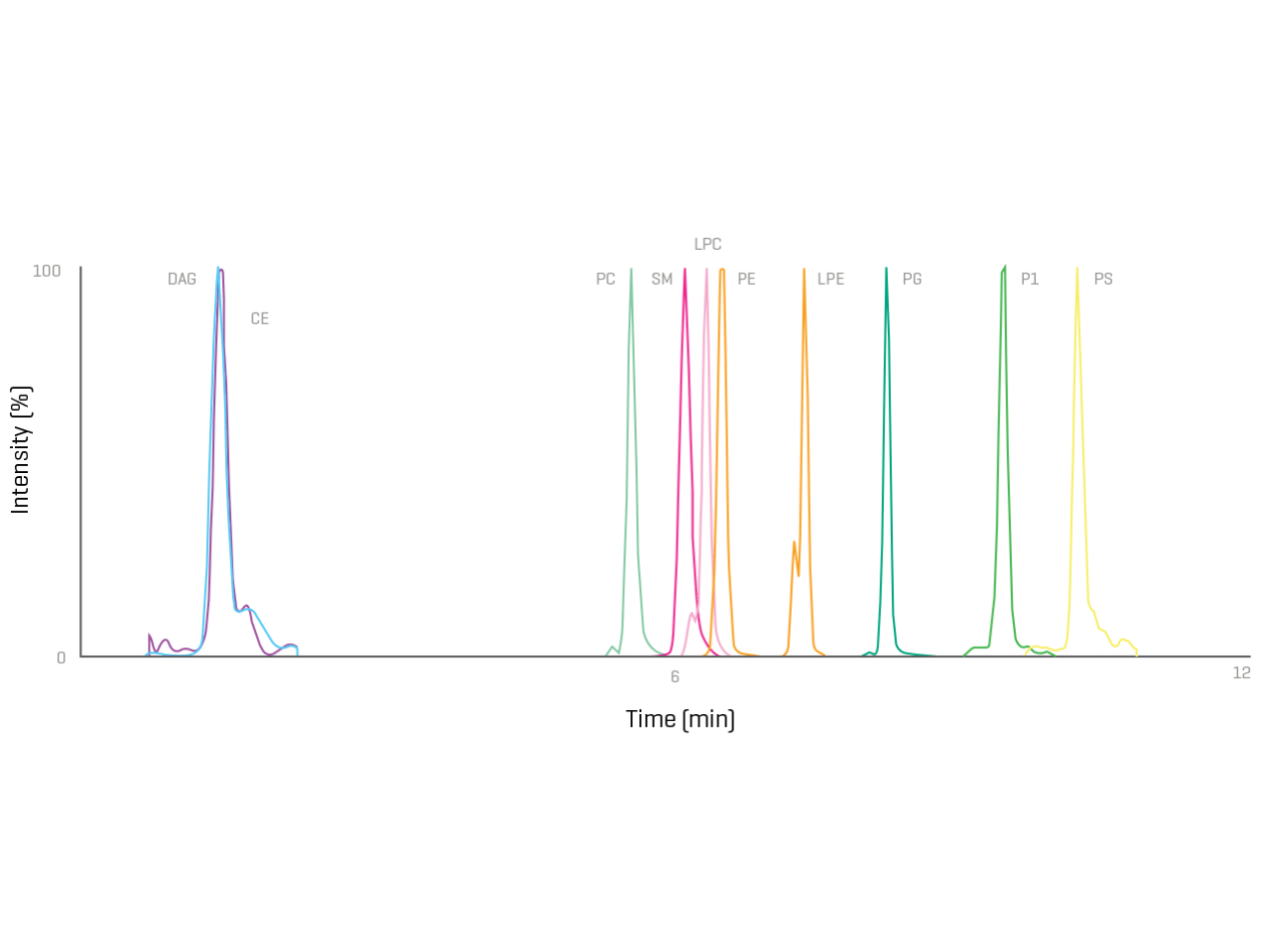
Take back your time with the ability to confirm and quantify hundreds of analytes in a streamlined manner.
Monitoring known lipid impurities and other critical components in lipid raw materials and LNPs is easier than ever before. Take control with intuitive, highly sensitive and reliable solutions.
- Achieve highly sensitive analyte detection independently of ionization polarity
- Easily obtain comprehensive results using intuitive data processing solutions
- Be confident with compliance-ready solutions that are robust and reliable
Solution
Lipid impurity quantitation
Suited for:
- Monitoring of known lipid impurities
- Superior sensitivity and robustness
All resources
-
Solution guide
Development of better LNP-based genetic medicines
Break through the boundaries of developing better LNP-based drugs. Take back your time with streamlined CE and MS workflows to learn more about your LNPs.
-
Technical note
Characterization of the ionizable lipid MC3 and its impurities
Break down frontiers of structural characterization of lipids and their impurities to develop better drugs. Dedicated solutions help your team confidently advance the next generation of therapies and vaccines.
-
Webinar
Adducts and N-oxides: Understanding LNPs for better mRNA-based drugs
Take control of lipid impurities! Learn how to save your team's time and resources by leveraging full solutions that enable you to break through the boundaries around the structural identification of lipids and their impurities.
-
Technical note
Comparison of lots of ionizable lipid ALC-0315
Create pathways to know more about your non-viral carrier. Discover solutions that reliably identify process-related lipid impurities from raw material and LNPs.
-
Q&A blog
Adducts and N-oxides: Understanding lipid nanoparticles (LNPs) for better mRNA drugs
Hear from expert Adam Crowe (Cytiva) about lipid impurities, the associated risk and how to develop better LNP-based drugs.
-
Technical note
Simultaneous identification and monitoring of lipids from a liposome
Set your own schedule by simultaneously monitoring lipids, using the robust and reliable quantitative data to continuously improve lipid-based delivery systems.
-
Webinar
Better mRNA-LNPs: encapsulation efficiency, mRNA integrity and purity, lipid N-oxides and beyond
To ensure the safety and efficacy of LNP-based drugs, comprehensive characterization of these sophisticated molecules—the lipids and the genetic cargo, such as messenger RNA (mRNA)—is paramount. This webinar explores the analytical challenges of characterizing mRNA-LNPs and a range of potential solutions.
Associated applications
Set your own schedule for CRISPR/Cas9 gene editing with innovative, intuitive analytical solutions. Unleash the potential of your Cas9 messenger RNA (mRNA), single guide RNA (sgRNA) and Cas9 proteins by understanding quality, purity and safety and assessing on/off target effects.
Break through barriers and extend the frontiers of messenger RNA (mRNA), self-amplifying RNA (saRNA) and circular RNA (circRNA) development with intuitive analytical solutions. Confidently innovate with high-quality, sensitive and accurate data to assess integrity, purity and critical quality attributes (CQAs).
Work in pursuit of a higher level of quality for characterization of synthetic oligonucleotides—such as single guide RNA (sgRNA) or prime editing guide RNA (pegRNA)—used for CRISPR/Cas9 gene editing applications, along with antisense oligonucleotides (ASOs), small interfering RNA (siRNA) and aptamer therapeutics.