High-throughput, targeted lipidomics in plasma using reversedphase chromatography
Thusitha Rupasinghe1, Jeremy Nicholson2, Nicola Gray2, Ben Crossett3, Xiao Suo Wang3, and Paul RS Baker4
1SCIEX, Australia, 2Australian National Phenome Centre, Murdoch University, Australia, 3University of Sydney, Australia, and 4SCIEX, USA
Published date: April 12, 2024
Abstract
In this technical note, the SCIEX 7500 system was used to perform high-throughput lipidomics analysis on human plasma samples using reversed-phase chromatography (RP-HPLC). Hydrophilic interaction chromatography (HILIC-HPLC) separates lipids by class and is typically used for lipid analysis to minimize inter-lipid class isobaric interference. However, this type of chromatography is very sensitive to pH, and retention times can fluctuate significantly. Also, co-elution of all members from a specific lipid class can cause duty cycle issues, particularly with triglycerides that have minimal retention on HILIC columns and can have >500 lipid species in targeted assays. RP-HPLC offers superior retention time stability, which can improve identification when used in concert with validated MRM transitions and primary reference and internal standards. Furthermore, spreading lipids more uniformly across the elution gradient can help mitigate duty cycle issues that can arise with HILIC-based approaches.
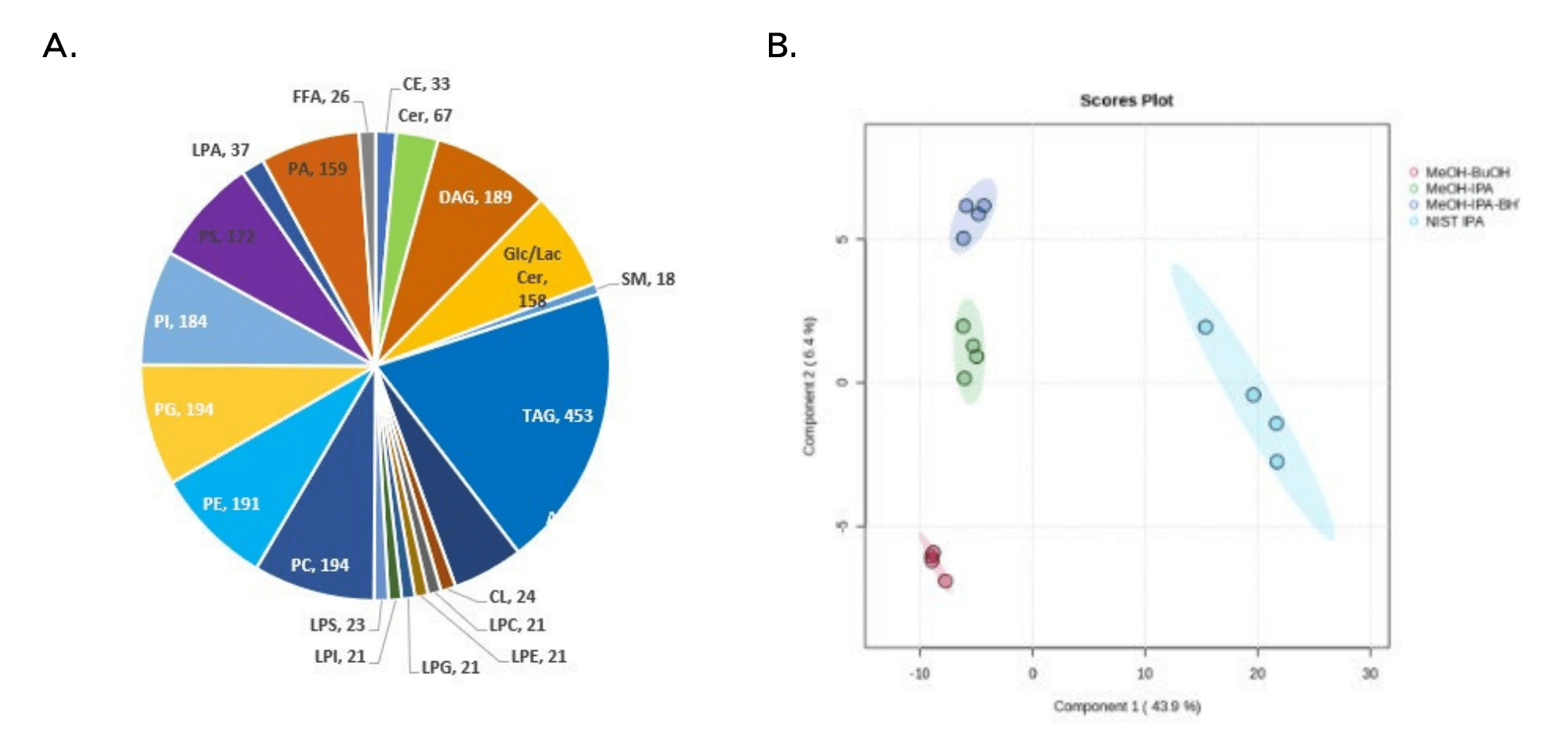
Here, the SCIEX 7500 system was used to measure lipid species identified at the fatty acid level in human plasma using a 15 min chromatographic separation. Because the workflow is intended to be relatively high throughput, a simplified, monophasic extraction technique was desired. Consequently, an optimal lipid extraction procedure was determined among 4 different monophasic lipid extraction techniques to find the one that generated the most consistent coverage of lipids during analysis. The data indicate that a butanol/methanol extraction (BUME method) provides the most reproducible monophasic extraction, and subsequent analysis using RP-HPLC mass spectrometry identified >1300 lipid species identified at the fatty acid level (Figure 1).
Figure 1. A. Scheduled MRM (sMRM) analysis of human plasma. Using RP-HPLC to resolve lipids in human plasma extracts, >1300 lipid species identified at the fatty acid level were detected among 21 lipid classes. B. Principal component analysis (PCA) of data sets derived using 4 different monophasic lipid extraction techniques. The BUME method provided the highest reproducibility among the 4 extraction methods tested
Key features of lipidomics analysis of human plasma using the SCIEX 7500 system with reversed-phase chromatography
- The SCIEX 7500 system using RP-HPLC can detect and quantitate > 1300 lipid species in human plasma
- The monophasic BUME extraction method provides rapid and reproducible lipid extraction from human plasma
- The rapid polarity switch time of the SCIEX 7500 system (< 5 ms) allows for analysis in both the positive and negative ion modes in a single injection to provide lipid detection and identification at the fatty acid level of structural specificity across all lipid classes
Introduction
Lipids play essential roles in cellular biology, including forming cell walls and intracellular organelles, mediating cell signaling events, and providing an energy depot. Cellular lipids are diverse and have a remarkable structural complexity within each lipid class. Their concentrations range from amol/ to nmol/mg protein, which represents 10-12 orders of magnitude linear dynamic range. The analysis of lipids is primarily mass spectrometry-based, and it is an essential component of omics-based research in systems biology.
The field of lipidomics is slowly and inexorably shifting from a purely discovery mode of analysis (i.e., untargeted analysis) to more quantitative analytical approaches. This evolution reflects the growing need to identify molecular changes in biosystems and provide potential biomarker concentrations in the context of standard reference values. Discovery experiments such as datadependent acquisition (DDA) by mass spectrometry can provide a relatively large number of candidate ions identified using dedicated software and, ideally, manual verification. A DDA experiment is designed to generate the broadest coverage possible. Ideally, only 1- 2 product ion spectra are acquired for each molecule, which does not provide sufficient data points for integration. Consequently, DDA experiments are limited to quantitating lipids at the precursor ion (i.e., MS1) level. Due to the extensive isobaric overlap among different lipid classes and regio- and fatty acid isomers within the same class, quantitating lipids at the MS1 level comes at the expense of structural specificity, and lipids are generally reported at the sum composition level of structural specificity. This data type denotes the class of an “identified” lipid but generalizes the fatty acid component to a sum of the total carbons and double bonds among the fatty acids along the glycerol backbone. For example, 1- palmitoyl-2-oleoyl-sn-glcerophosphocholine, which denotes class, fatty acids, and their positions along the glycerol backbone, is reduced to PC 34:1, which lacks essential fatty information pertinent to metabolism and likely represents >50 individual lipid molecular species, depending on the fatty acids present in the biological system being analyzed.
A targeted approach to lipid analysis using a multiple reaction monitoring (MRM) scan mode provides lipid identification at the fatty acid level, and it enables compounds to be quantitated absolutely, accurately or relatively, depending on the internal standard strategy employed (1-4). Typically, targeted analysis is done using a triple quadrupole mass spectrometer, which is ideal for targeted quantitation due to its high sensitivity, fast speed, and MS/MS-based specificity. The SCIEX 7500 system can analyze a single MRM in as little as 4 ms (1 ms dwell time and 3 ms pause time), enabling as many as 250 MS/MS events each second. This speed is essential considering the SCIEX comprehensive targeted lipidomics panel targets over 2300 lipid molecular species. Lipid analysis can be performed using various types of chromatographic separation, each with advantages and disadvantages. HILIC-based chromatography separates lipids by class, which solves the significant problem of inter-class isobaric interference but raises the issue of duty cycle with so many lipids within each class coeluting. Reversed-phase chromatography separates lipids based on hydrophobicity, which is directly related to the fatty acid chain length. This allows lipids to be more evenly distributed along the chromatographic gradient, but the data analysis is subject to a greater level of false positive results due to the isobaric interference among different lipid classes. However, with a careful notation of retention times, the use of authentic standards, and the careful selection of MRM transitions, RP-HPLC is an excellent means to resolve lipids from complex mixtures.
Herein, Lipids were extracted from human plasma using 4 different monophasic extraction techniques to identify the method that provided the most consistent results. These extracts were resolved using RP-HPLC and analyzed using a SCIEX 7500 system in the MRM scan mode. The method was designed to accurately detect and quantitate more than 2300 lipid molecular species among 21 lipid classes. The lipids were identified and quantitated at the fatty acid level.
Materials and methods
Materials: Organic solvents, all Optima-LCMS grade or better, were purchased from Thermo Fischer Scientific (Sydney, Australia). LC-MS grade water was generated in-house using a Milli-Q IQ 7000 system (Merck Millipore). Ultimate SPLASH mix Lipid Internal Standards Kit, SPLASH LipidoMIX internal standards, Lyso PI 17:1, Lyso PG 17:1 and Lyso PS 17:1 were obtained from Avanti Polar Lipids (Sigma-Aldrich, North Ryde, NSW, Australia). A working solution of the Ultimate SPLASH mix Internal Standards Kit, SPLASH LipidoMIX and Avanti Polar Lipids internal standards was created by diluting all internal standards 1:200 with propan-2-ol (IPA).
Sample preparation: Plasma samples were obtained from NIST (NIST SRM 1950 human plasma), and 10 μL of plasma was extracted by the addition of 90μL of the organic solvent containing stable isotope-labeled internal standards. Four monophasic extraction solvents were evaluated: butanol:methanol (BUME, 1:1 (v/v)) containing 10 mM ammonium acetate, propan-2-ol (IPA), propan-2- ol:methanol (IPA:MeOH with BHT, 1:1 (v/v)) and propan-2- ol:methanol (IPA:MeOH, 1:1 (v/v)). Four technical replicates per extraction protocol were prepared for analysis. Samples were diluted with each solvent prior to 1-minute vortex and centrifugation at 14000 g for 10 min. The supernatant (90 μL) was collected and transferred to 2 mL HPLC vials with glass inserts.
Chromatography: Reversed-phase chromatographic separation was performed using a SCIEX Exion LC system (SCIEX, Concord, CA) comprised of a binary ultra-high pressure gradient pump with degasser, a thermostated autosampler, and a column oven. Separation was performed using a Phenomenex Kinetex C18 Column (2.6 µm, 100 AÅ , 100 x 2.1 mm) held at 55°C. Mobile phase A was water/acetonitrile/propan-2-ol (50/30/20, v/v/v) containing 10 mM ammonium acetate and mobile phase B was propan-2- ol/acetonitrile/water (90/9/1, v/v/v) containing 10 mM ammonium acetate. The flow rate was 0.4 mL/min with gradient elution starting at 10% B. The gradient profile is shown in Table 1. The injection volume was 5 μL,, and samples were kept in the autosampler at 10 °C.
Table 1. Gradient elution profile
Mass spectrometry: Lipid detection was performed using a SCIEX QTRAP 7500 system (SCIEX, Concord, CA) with electrospray ionization using the OptiFlow Pro Ion Source and operated in the high mass mode with polarity switching. The system parameter settings are listed in Table 2, and lipid class-specific collision energy (CE) settings are listed in Table 3. Time-scheduled multiple reaction monitoring (sMRM) was used for data acquisition of 2291 transitions that include 2215 lipid species across 22 different classes and 76 internal standards.
Table 2: SCIEX 7500 system parameter settings
Table 3: Collision energy (CE) settings for lipid classes in the SCIEX 7500 system
Data processing: LC-MS operation and data acquisition and data processing were performed using SCIEX OS 3.1.0 software (SCIEX, MA, USA). Principal component analysis (PCA) was performed using MarkerView software, which is an extension available in SCIEX OS software.
Results and Discussion
MRM transitions were curated to cover the most abundant lipid classes in human plasma, including PC/LPC, PE/LPE, PS/LPS, PI/LPI, PG/LPG, DAG, TAG, FFA, CE, CER, DCER, HCER, LHCER, CL, ACar and SM (Table 3). The targeted scheduled MRM (sMRM) list was developed to include 2291 lipid molecular species, with 1235 transitions in negative and 1056 in positive ion modes, respectively. Fast polarity switching is essential for this workflow to identify lipids at the fatty acid level of structural specificity. In the negative ion mode, phospholipids fragment to lose their fatty acyl chains, allowing for fatty acid identification within the molecule. In the positive ion mode, the fatty acid composition of CE, DAG, and TAG lipid species can be determined using MRM transitions designed to accommodate the associated neutral loss of an ammoniated fatty acyl chain.
One challenge to using RP-HPLC to resolve complex lipid mixtures is the isobaric overlap of lipids from different classes throughout the gradient elution. The lipidome is believed to comprise over 150,000 lipid molecular species, most of which are found within a narrow mass range (~ 800 Da). Consequently, isobaric overlap is common, and multiple lipid species with the same or nearly the same mass can coelute at any given point along the gradient. Many of these intra-class isobars also share common acyl chain fragment ions, so the specificity normally associated with MRM scan modes may not apply. Thus, it is very important to ascribe accurate retention times to lipid species to minimize false positive data. RP-HPLC is noted for robust, consistent retention times that are not unduly affected by slight variations in pH, as is the case with HILIC-HPLC. Figure 2 shows the reproducibility of the RP-HPLC method. Using the same column, 10 µL extraction equivalents of NIST plasma lipid were anlayzed. Four technical replicates for each extraction protocol were analyzed. Even, though the 4 lipid extraction injectates were of different solvent compositions, the reproducibility of chromatographic separation is consistent throughout the 15-minute gradient.
Figure 2. Reproducibility of the reverse phase chromatography for plasma lipid extracts using four different extraction protocols. The total ion chromatograms (TIC) from 4 technical replicates of 4 separate sample types were overlaid (n=16) to demonstrate the highly reproducible chromatographic elution of lipid from the RP-HPLC column
Reversed-phase chromatography separates lipids by their hydrophobicity, which is primarily related to the acyl chain length. However, the presence of double bonds can also affect retention times. Figure 3 shows the effects of double bonds on a series of triacylglycerol (TAG) molecules with the same sum composition of carbons among their respective fatty acyl chains. The retention time of these molecules is inversely related to the number of carboncarbon double bonds, with the elution order of TAG 54:8, TAG 54:7, TAG 54:6 and TAG 54:5. Because the number of primary reference standards is very low compared to the number of lipid molecular species in plasma, this trend should be considered during data review and can help reduce false positive results.
Figure 3. Effect of of acyl chain double bonds on retention time. TAG species with the sum composition of 54 carbons display a retention timedependence on the total number of carbon-carbon double bonds, with the relative retention time increasing as the number of double bonds decreases. In this experiment, lipids were extracted from plasma using the BUME protocol.
The specificity of this analytical method is achieved by effective chromatographic resolution and the use of curated MRM transitions. There are numerous isobars among lipids, not only within the same class but also from different lipid classes. Figure 4 shows the extracted ion chromatograms (XICs) of multiple isobaric lipid molecular species. Figure 4A shows distinct elution profiles of lipids from three different lipid classes. They are distinguished not only by their respective retention times, but also by their unique MRM transitions. Combined, precise retention times and curated MRM transitions provide a high level of accuracy in the measurement of lipids in plasma. Identifying isobars within the same lipid class relies more on unique MRM transitions than retention times. Figure 4B shows multiple isobars for PC and PE. The sum composition species PC 34:1 can be further defined using MRM transitions to distinguish the isobars at the fatty acid level. Three peaks are shown: PC (14:0_20:1), PC (16:0_18:1), and PC (16:1_18:0). These isomers essentially co-elute but can be detected by using distinct MRM transitions.
Figure 4. Elution profiles of lipid isobars. A: Lipid isobars from three different lipid classes can be resolved by chromatography and unique MRM transitions. B: In situations where there is co-elution of lipid isomers, as is the case with PC (14:0_20:1), PC (16:0_18:1), and PC (16:1_18:0), the accuracy of lipid identification is primarily reliant on the use of unique MRM transitions. In these experiments, lipids were extracted from plasma using the BUME protocol.
One of the goals of this study was to evaluate multiple different monophasic extraction techniques to find the one that yielded the best coverage and reproducibility. Using the targeted RP-HPLC ESI MS/MS method described above, 4 different extraction procedures were used, and the results were evaluated by principal component analysis (PCA; Figure 1B) using MarkerView software. Based on the PCA analysis, the BUME extraction protocol provides the highest reproducibility. The addition of BHT did not significantly affect the number of detected lipid species, and its inclusion may help reduce the adventitious oxidation of plasma lipids. The IPA extraction protocol showed significant differences in the lipid molecular species profile from those obtained using the BUME, MeOH-IPA, and MeOH-IPA with BHT extraction protocols, as shown via a heatmap in Figure 5. Here, the x-axis shows the 16 individual runs, grouped as 4 technical replicates for each extraction protocol; the y-axis depicts each individual lipid species detected; and the color indicates the relative concentrations of these species, as detailed in the legend. The IPA extraction is aligned to the far left of the heat map, and the differential colors show a clear difference in approximately 60% of the extracted lipids from the other three techniques. No significant differences in the extraction efficiency at the lipid molecular species level were observed for the other three extraction methods.
Figure 5. Heatmap of the plasma lipid profile from four extraction protocols. Plasma lipid profiles using BUME, MeOH-IPA and MeOH-IPA with BHT show similar extraction efficiencies at the lipid molecular species level. In contrast, significant differences are apparent in the lipid profiles generated using IPA extraction protocol (far left 4 columns)
The extraction efficiencies for the 4 different extraction protocols were also assessed at the lipid class level (Figure 6). The total peak areas for all lipid species within each lipid class were compared for each extraction protocol and are presented as the log10 value of the total peak area per class. As illustrated, Glycerophospholipids show similar extraction efficiencies using all four lipid extraction protocols. As expected, MeOH-IPA extraction protocol showed no difference in extraction efficiencies, with and without BHT added as an antioxidant. Both BUME and IPA extraction protocols provide higher extraction of lipid molecular species in lipid classes of TAG and CE with lower extraction efficiency of lipid species in DAG lipid class, compared to the extraction protocols of MeOH-IPA and MeOH-IPA-BHT. Based on these data, the BUME, MeOH-IPA, and MeOH-IPA with BHT monophasic techniques all appear to provide similar extraction efficiencies for lipids at the molecular species and class levels. However, the IPA extraction method appears to differ significantly from these three when evaluated at the lipid molecular species level and may not be an appropriate method by comparison.
Figure 6. Lipid class comparison of the extraction efficiency between 4 different monophasic lipid extraction techniques. The peak areas of the individual lipid molecular species detected using the RP-HPLC approach were summed for each lipid class and presented in histogram format to compare the 4 extraction techniques. At the lipid class level, there does not appear to be significant differences in their extraction efficiencies
The lipid data was collected by injecting 4 replicate injections from each extraction protocol to evaluate the qualitative reproducibility of the data. The reproducibility of the sMRM RP-HPLC methodology was assessed by calculating the % CV of the peak area for each set of extraction replicates. Data illustrated in Figure 1A provides the number of lipid species detected per lipid class in a 1 µL plasma extract equivalent. The data show the number of lipid species per class with a 30% CV or less and represent ~1300 lipids across 21 lipid classes.
In summary, this technical note describes the methodology for the efficient extraction of lipids from plasma using a monophasic extraction technique and detecting more than 1,300 accurately identified lipid molecular species. To address the potential of false positive detection of lipids that can occur during untargeted lipid analysis via RP-HPLC, a targeted lipidomics approach was used that depends on reproducible chromatographic retention times and unique MRM transitions to enable the accurate detection of lipid molecular species identified and quantified at the fatty acid level.
Conclusion
- The SCIEX 7500 system has the speed and sensitivity to measure more than 2300 lipid molecular species identified at the fatty acid level
- The use of highly reproducible RP-HPLC separation and unique MRM transitions enables accurate lipid identification
- Targeted lipid analysis allows for quantitation of lipid molecular species, providing important context for biological studies
- Multiple monophasic extraction techniques were evaluated for extraction efficiency and reproducibility; the BUME extraction protocol yielded the most consistent results with the broadest coverage
References
- Ryan M, James A,G, Lawler N G, Fear M, W, et al. (2023) Comprehensive Lipidomics Workflow for Multicohort Population Phenotyping Using Stable Isotope Dilution Targeted Liquid Chromatography-Mass Spectrometry.
https://doi.org/10.1021/acs.jproteome.2c00682
- Broad Lipid Profiling using a High-Throughput Targeted LC-MRM Method (sciex.com)
- Huynh K, Barlow CK, Jayawardana KS, Weir JM, Mellett NA, Cinel M, et al. (2018) High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chemical Biology.
https://pubmed.ncbi.nlm.nih.gov/30415965
- Thomas G. Meikle, Kevin Huynh, Corey Giles, and Peter J. Meikle. (2021) Clinical lipidomics: realizing the potential of lipid profiling.
https://www.jlr.org/article/S0022- 2275(21)00109-7/pdf
 Click to enlarge
Click to enlarge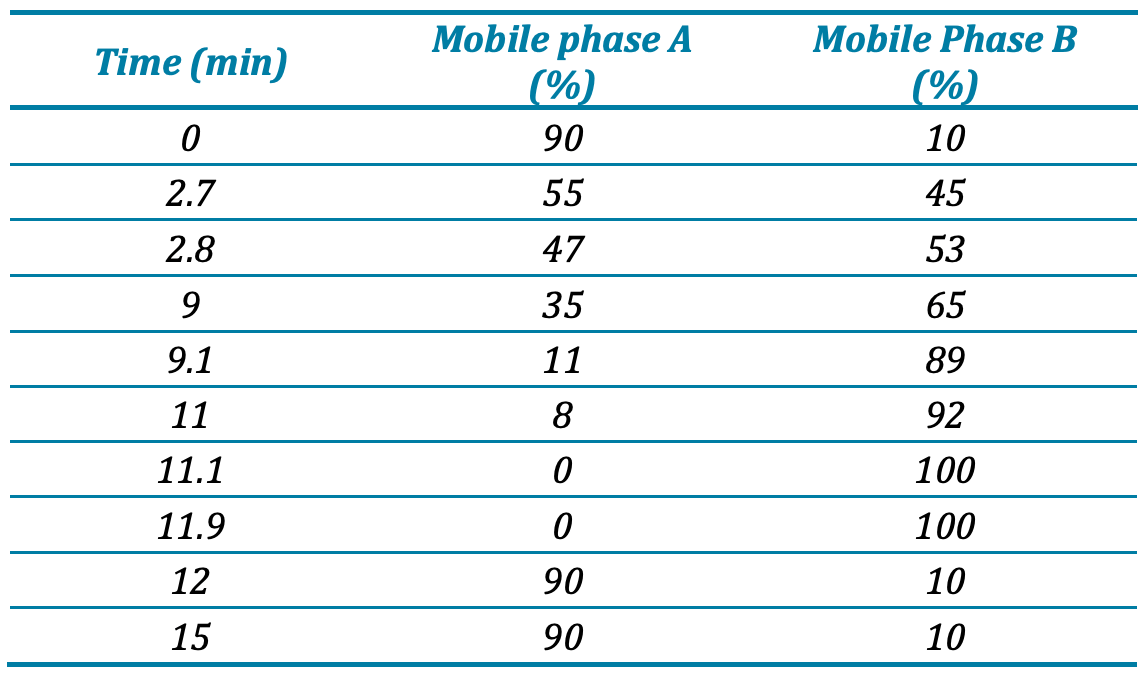 Click to enlarge
Click to enlarge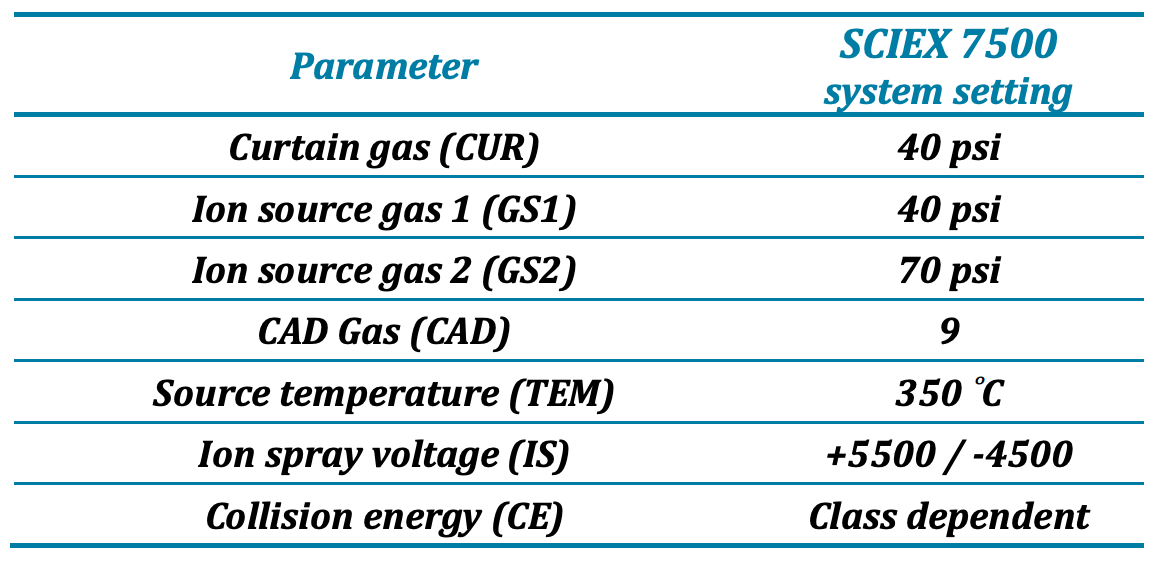 Click to enlarge
Click to enlarge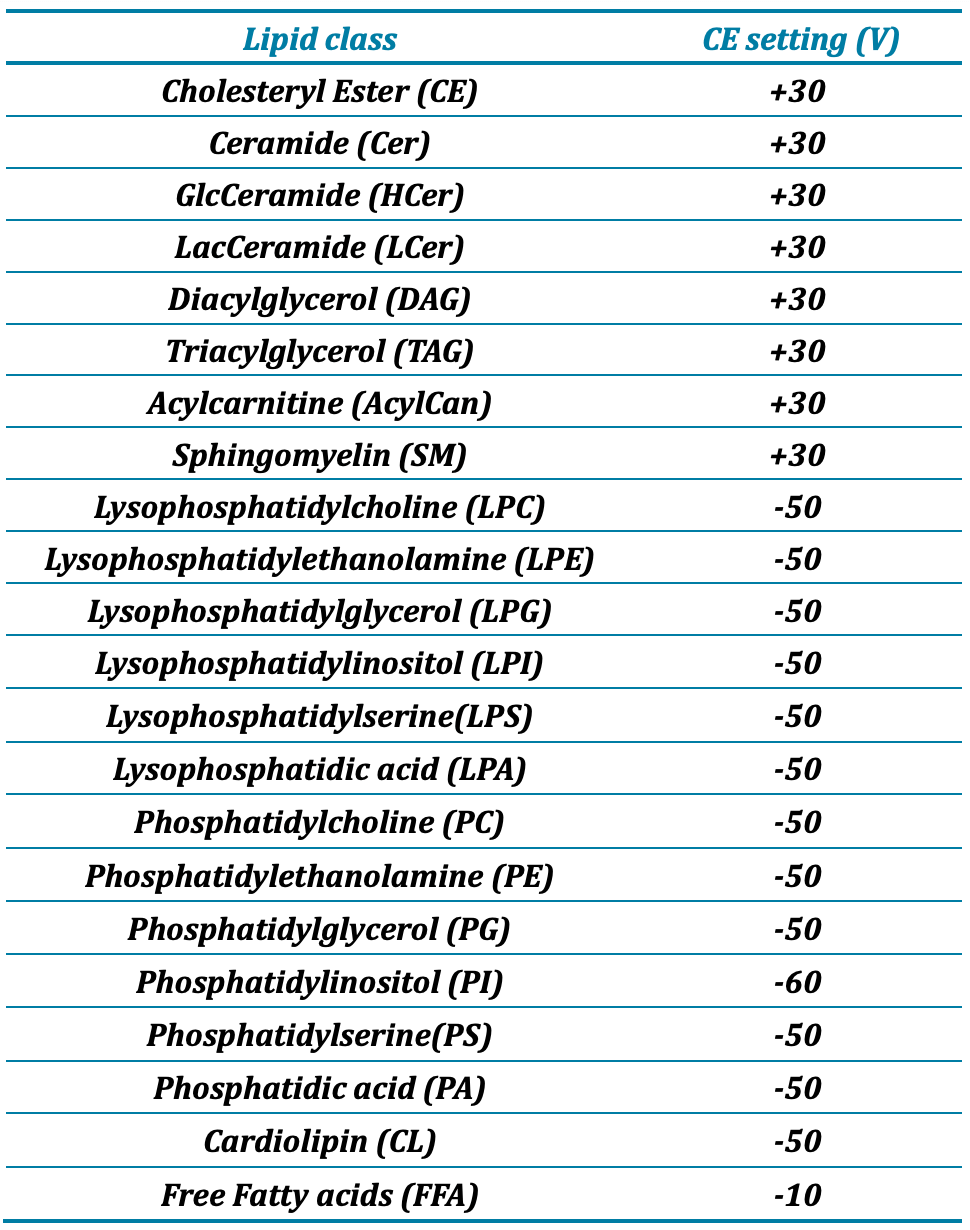 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge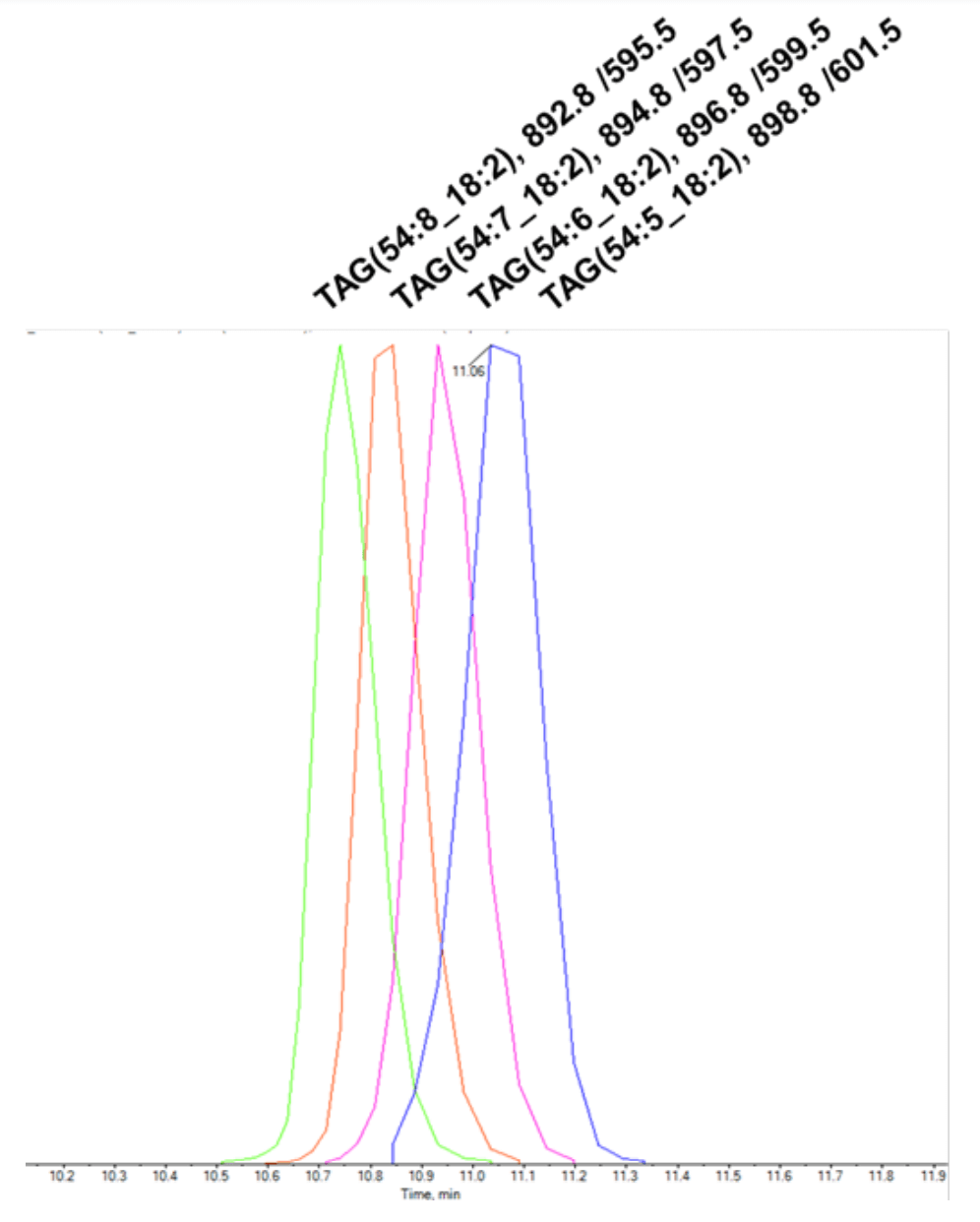 Click to enlarge
Click to enlarge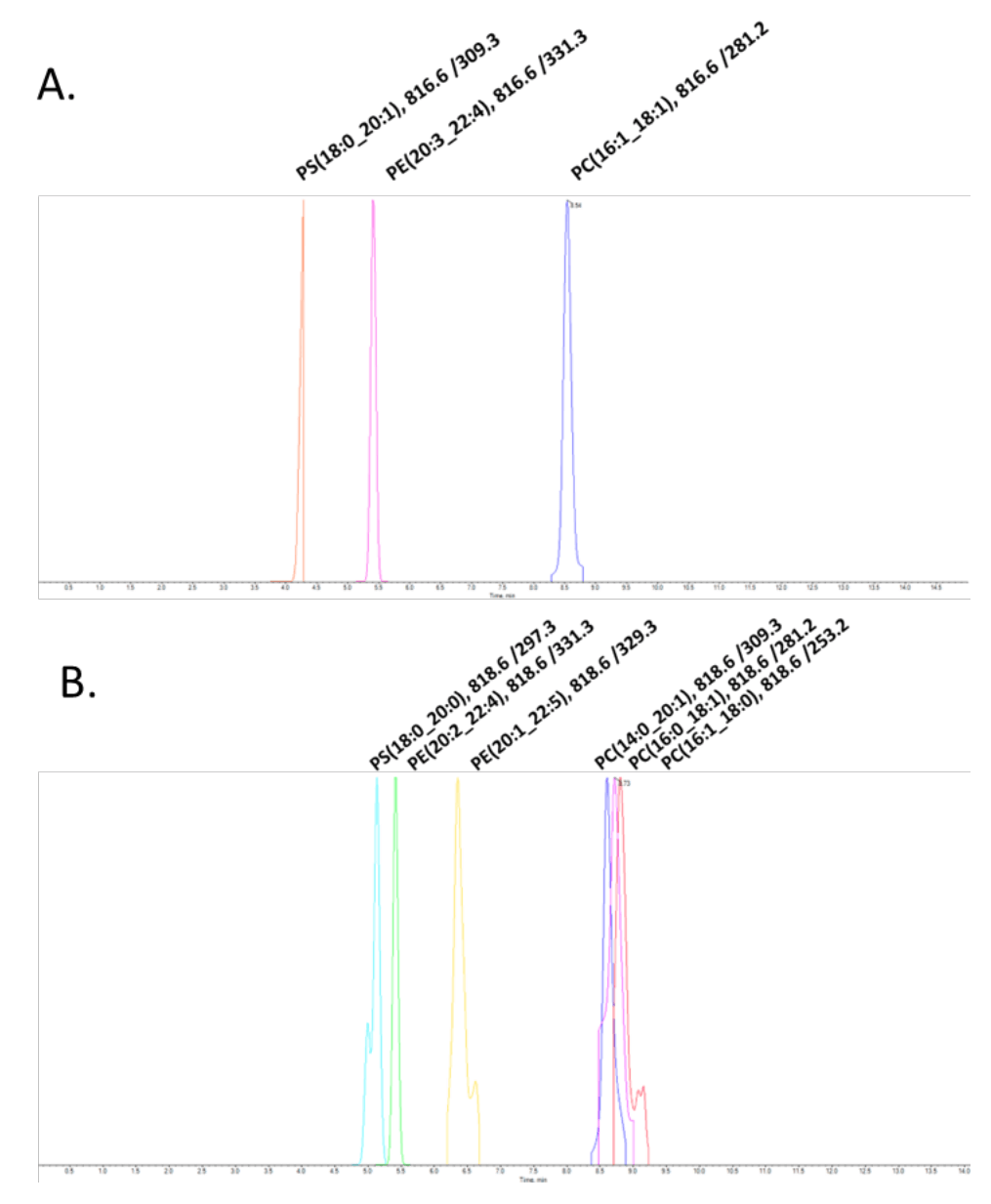 Click to enlarge
Click to enlarge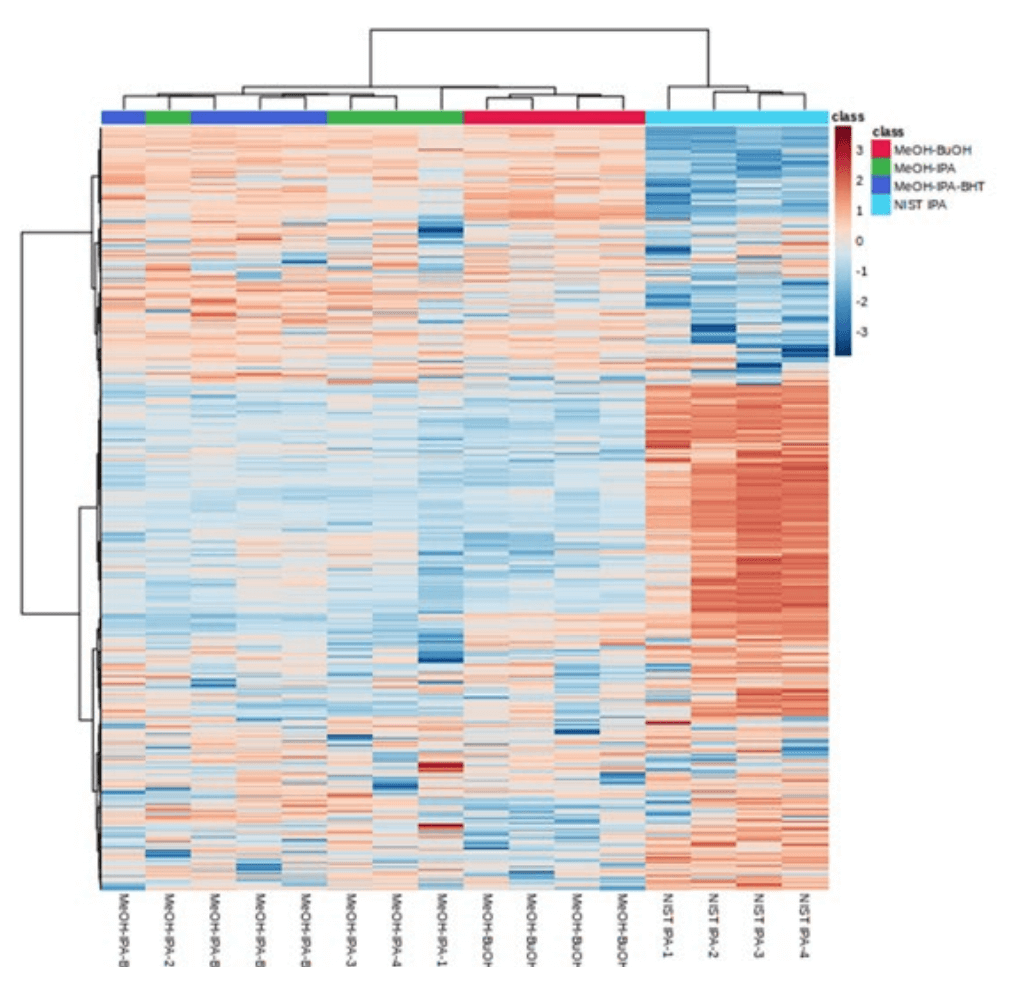 Click to enlarge
Click to enlarge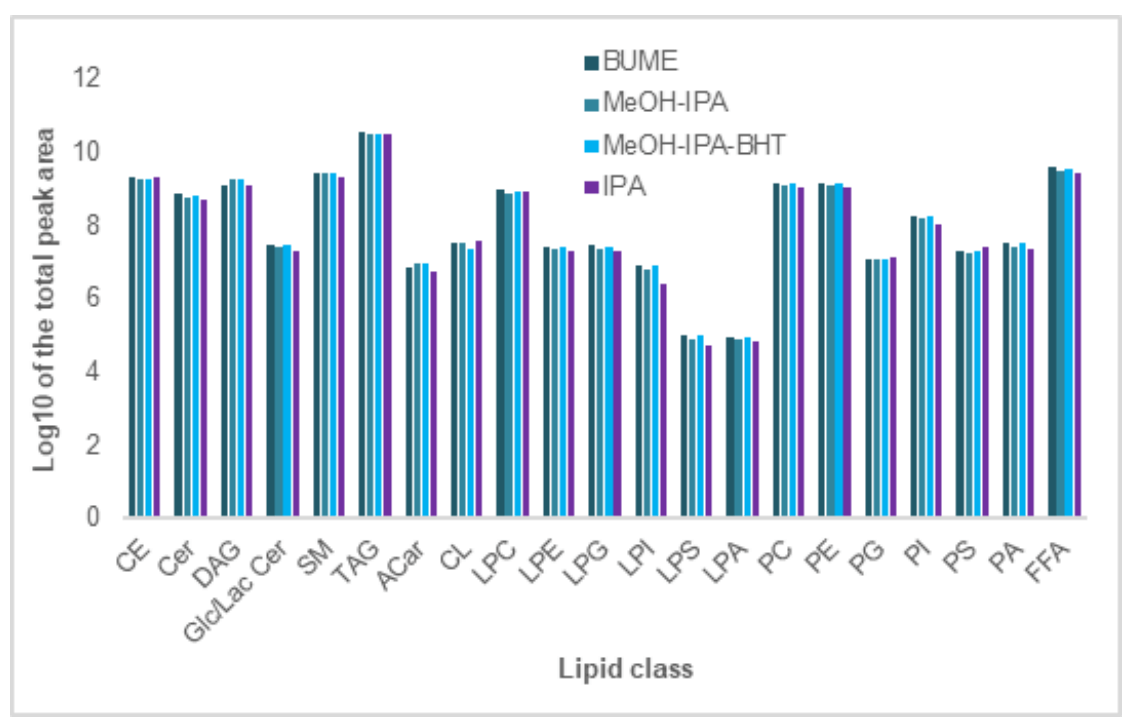 Click to enlarge
Click to enlarge