Quantification of infliximab in human serum by LC-MS/MS
Using the Citrine® Triple Quad MS/MS System
J. Grace van der Gugten1, Daniel T Holmes1, Dan Blake2
1Department of Pathology and Laboratory Medicine, St. Paul’s Hospital, Vancouver, BC, Canada; 2SCIEX, Alderley Edge, UK
Abstract
Here, an LC-MS/MS based approach for the quantification of infliximab in human serum has been developed using the SCIEX Citrine LC-MS/MS system. Using an automatable workflow for denaturation and digestion and a very short LC-MS run, very good performance was achieved. Comparisons between this assay and previously developed MS-based assays showed very good agreement across the concentration ranges assessed.

Introduction
Quantification of monoclonal antibodies (mAbs) in biological fluids is important during all stages of antibody-based drug development. Infliximab (IFX) is one such antibody-based drug. It acts by neutralizing the biological activity of TNF-α. IFX is commonly used in the management and research of several autoimmune conditions. Accurate quantification of circulating levels is of high importance.
Traditionally, immunoassays are used for quantification of such analytes. Recently, liquid chromatography-mass spectrometry has been adopted because of its high selectivity, accuracy and precision. Mass spectrometry is a potential route for standardization of measurement due to its many advantages over established methodologies.
The workflow for development of a mass spectrometry-based approach for quantification of large molecules such as mAbs has long been established. Unique signature peptides are selected based on criteria such as digestion efficiency, stability after digestion, and chromatographic behavior and MS-MS sensitivity. They are then measured using LC-MS in MRM mode. Sensitivity of the LC-MS instrumentation and methodology can be an important factor in determining complexity of sample preparation procedures. An LC-MS/MS-based approach is presented here for the quantification of IFX in human serum.
Key features of the Citrine® Triple Quad MS/MS System for the analysis of infliximab
- Rapid, robust approach allows direct detection of IFX in plasma with automatable sample preparation and fast chromatography
- Targeted MRM workflow allows sensitive detection with high selectivity, reduced matrix effects and higher confidence in results
- Advanced ionization and source technologies allow for robust and reproducible analysis with minimal operator intervention
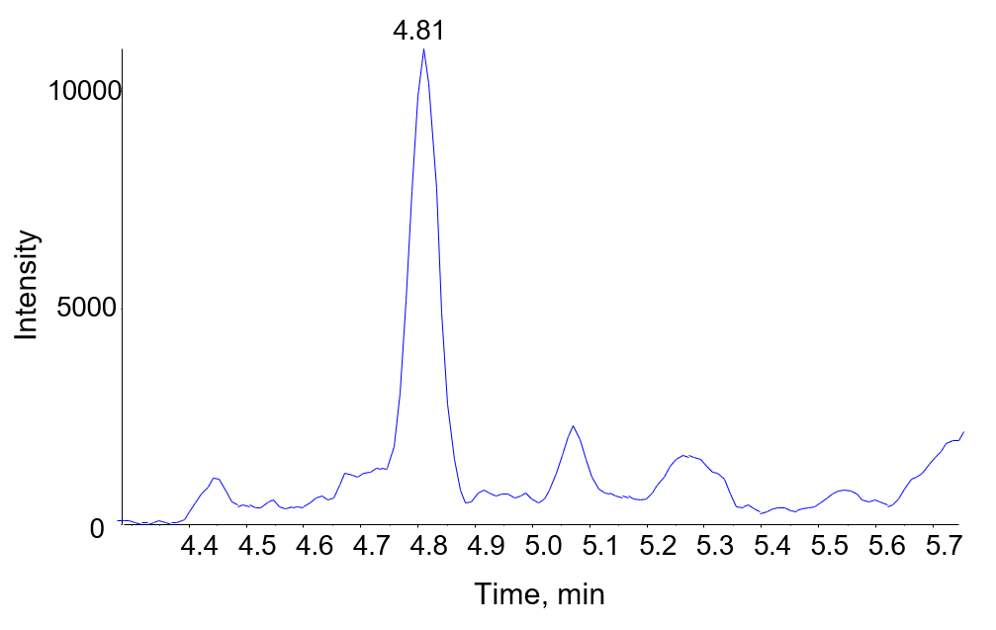
Figure 1. Chromatogram of Infliximab in human serum. Extracted ion chromatogram for a peptide from Infliximab in human serum, analyzed as described and quantified at 1.56 µg/mL.
Methods
Sample preparation: 25 µL of 0.08 µg/mL heavy labelled peptide (internal standard), 100 µL ammonium bicarbonate and 50 µL trifluoroethanol was added to 10 µL of serum. Following incubation at 55°C with vortex mixing at 1050 rpm for 30 minutes, 250 µL deionized water and 50 µL of 1 mg/mL trypsin was added. The sample was then incubated at 37°C with vortex mixing at 1050 rpm for 1 hour. Incubation was stopped with the addition of 10 µL concentrated formic acid. Following a final vortex mix, 20 µL of the sample was analyzed by LC-MS/MS.
Chromatography: Chromatographic separation was achieved using an Aeris 3.6um PEPTIDE XB-C18 100A, LC column 50x2.1mm (Phenomenex, PN 00B-4507-AN) with mobile phases composed of water (mobile phase A) and acetonitrile (mobile phase B), both containing 0.1 % (v/v) formic acid. A gradient method was employed at a flow rate of 0.35 mL/min.
Mass spectrometry: Mass spectrometry analysis was performed using a Citrine® Triple Quad MS/MS System, operating in positive electrospray mode. Compound-dependent parameters were optimized for the IFX light chain peptide and its corresponding internal standard. A total of 3 MRM transitions were analyzed for the IFX light chain peptide and 2 MRM transitions for the IS. IgG1 was also included as a monitor of the digestion process. Details of parameters used are given in Table 1.
Data processing: Quantification was carried out using Analyst® MD Software 1.6.3. Standard curves were generated using linear fits with a 1/x weighting.
Table 1. Mass spectrometry parameters for target peptides.
Analytical sensitivity
Analytical sensitivity was investigated with a series of calibration standards and QC samples prepared in matrix and processed as described. Figure 2 shows a 0.5 µg/mL IFX QC sample (IFX light 1 MRM transition shown) with a signal:noise (based on a peak-to-peak algorithm) of 11:1.
Analytical linearity
A series of calibrators were prepared in pooled blank serum from stock solutions of IFX (brand name: Remicade®) at 10, 1 and 0.1 mg/mL in deionized water. The calibration curve consisted of 8 calibration points over an analytical range of 0.5-40 µg/mL in serum. Figure 3 shows a calibration curve for the primary MRM transition (IFX light 1) analyzed as described. The calibration standards were extracted once and injected twice, at the beginning and end of each analytical run. Linearity (r2) was calculated at 0.9984.
Figure 2. Chromatogram of a 0.5 µg/mL infliximab in serum. The QC sample which was processed as described was analyzed by LC-MS/MS. The S/N was found to be 11 (peak to peak algorithm).
Figure 3. Calibration curve for IFX using the primary MRM transition (IFX light 1). Calibration curve showed excellent linearity from 0.5 to 40 µg/mL in human serum with an r of 0.9992 (r2 of 0.9984).
Precision
Assay precision was calculated from QC samples analyzed in quintuplicate over a series of 5 analytical runs (n=25). The QC samples were prepared from pooled, pre-quantified samples diluted appropriately with pooled blank matrix to produce three concentration sets at 0.5, 5 and 25 µg/mL. Results and statistical analysis are shown in Table 2.
Table 2. Precision of assay.
Comparison with existing MS-based assays
To show acceptable performance when compared with established assays, two series (Series A, n=63 and Series B, n=55) of pre-analyzed samples were processed by the proposed methodology and compared with results already generated. Series A was compared with a previously established in-house mass spectrometry-based assay and Series B was compared with an external mass spectrometry-based assay. Figures 4 and 5 show Passing-Bablok regression plots for these comparison data sets.
Figure 4. Series A results — comparison to previous in-house MS assay. Passing Bablok plot of comparison data generated comparing the LC-MS/MS assay generated in this work to an established in-house mass spectrometry assay (Series A, n=63).
Figure 5. Series B results — comparison to previous external MS assay. Passing Bablok plot of comparison data generated comparing the LC-MS/MS assay generated in this work to an established external mass spectrometry assay (Series B, n=55).
Conclusions
The data were generated from a workflow for the sensitive quantification of IFX in human serum. The automatable workflow consisted of denaturation and tryptic digestion, followed by a short (9 min) LC-MS analysis.
The assay demonstrated the following performance parameters:
- At a level of 0.5 µg/mL in serum matrix, S/N compute using the peak-to-peak algorithm was shown to be 11:1
- Good linearity (r2 > 0.998) was shown over a range of 0.5–40 µg/mL in serum matrix
- Accuracy and precision, assessed using diluted pre-analyzed samples at three discreet concentrations, was shown to be between 96 and 107% accurate with a %CV of ≤8.6% for all concentrations analyzed.
- Comparisons of previously analyzed samples between this proposed workflow and established MS-based assays (in-house and external) for the quantification of IFX show good agreement across all concentration ranges analyzed
References
- van der Gugten JG, Bressler B, DeMarco ML, (2019) An automated mass spectrometric blood test for therapeutic drug monitoring of infliximab, Clinical Mass Spectrometry, 12 16-22.
- Willrich MAV, Murray DL, Barnidge DR, Ladwig PM, Snyder MR, (2015) Quantitation of infliximab using clonotypic peptides and selective reaction monitoring by LC–MS/MS, International Immunopharmacology, 28 513-520.
 Click to enlarge
Click to enlarge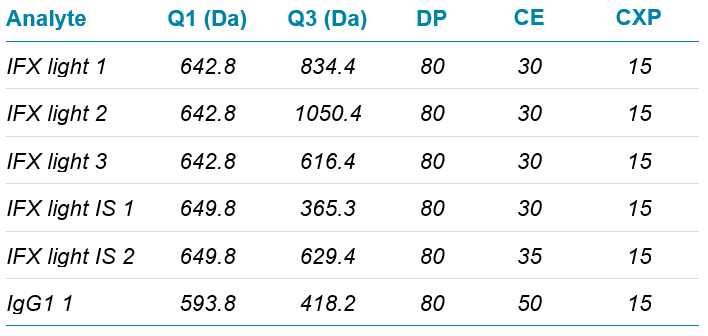 Click to enlarge
Click to enlarge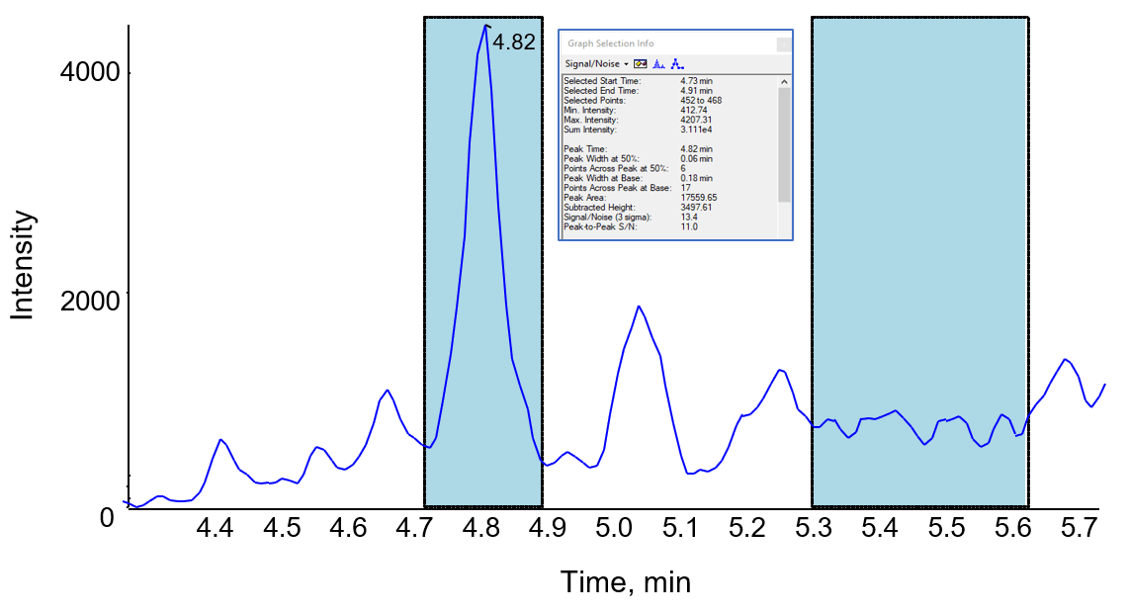 Click to enlarge
Click to enlarge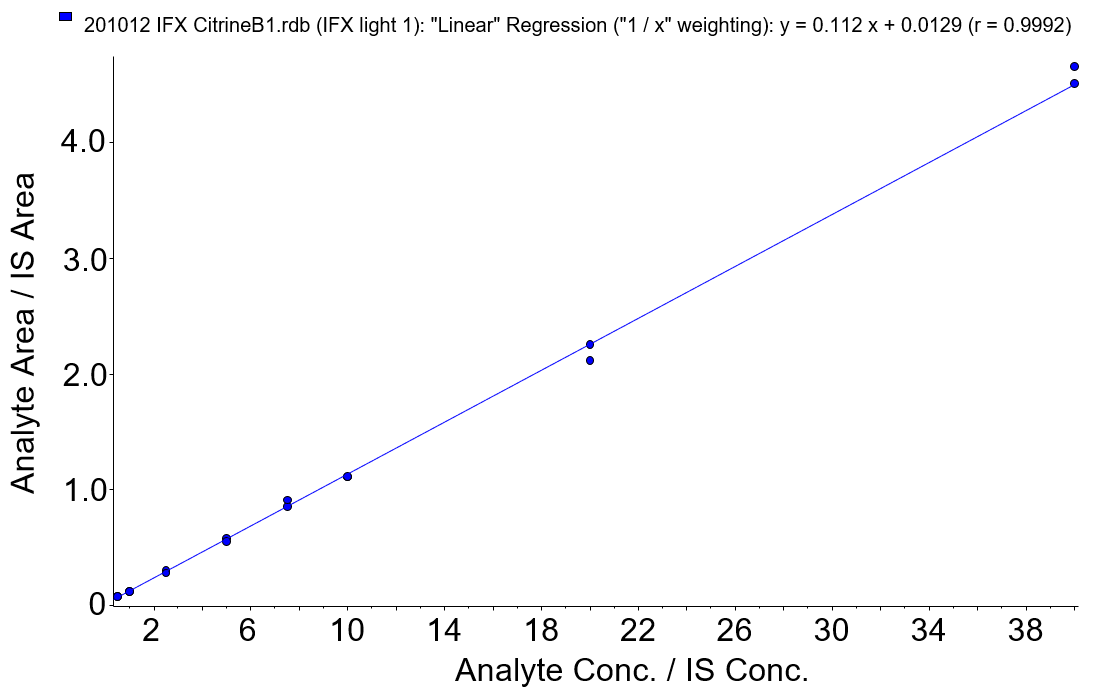 Click to enlarge
Click to enlarge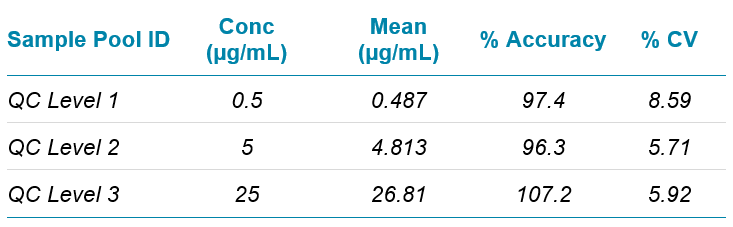 Click to enlarge
Click to enlarge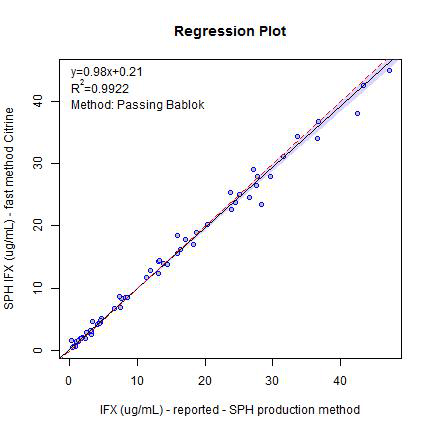 Click to enlarge
Click to enlarge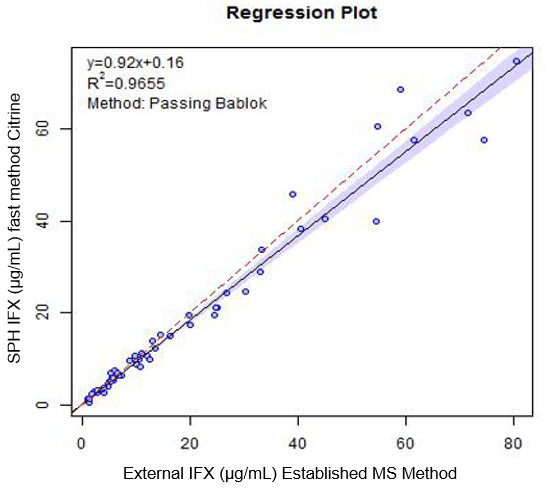 Click to enlarge
Click to enlarge