The amazing complexity of cellular networks is still not fully understood and therefore modifying a single gene to treat a patient can have various effects that need to be determined. Answering the questions about what is happening at the cellular level requires an untargeted and very sensitive approach. State-of-the-art, highly sensitive Zeno SWATH data-independent acquisition (DIA) can determine off- and on-target effects with unprecedented depth and short injection-to-injection times that allow for necessary throughput. Here, Hugo Gagnon will discuss experimental design, depth of analysis, target coverage and statistical challenges to help biopharmaceutical drug developers ensure safe and efficient gene therapy products.
Extraordinary science with Hugo Gagnon
At Allumiqs, Hugo Gagnon investigates high-throughput proteomics to validate new drug development

Exploring high-throughput proteomics for new drug development
Key takeaways
- Using LC-MS based proteomics for high-throughput drug discovery is feasible
- High-throughput proteomics offers an in-depth, unbiased approach to overcome the limitations of common methods for drug development (for example, PROTACs and CRISPR)
- High-throughput proteomics requires careful project design for optimization
- Depth of coverage vs. acquisition speed
- Robustness vs. study design
- Technical variability (instrument, biological, processing)
- Faster gradients can be beneficial beyond their use for high-throughput proteomics by supporting better chromatography with sharper and taller peaks with improved signal-to-noise ratio
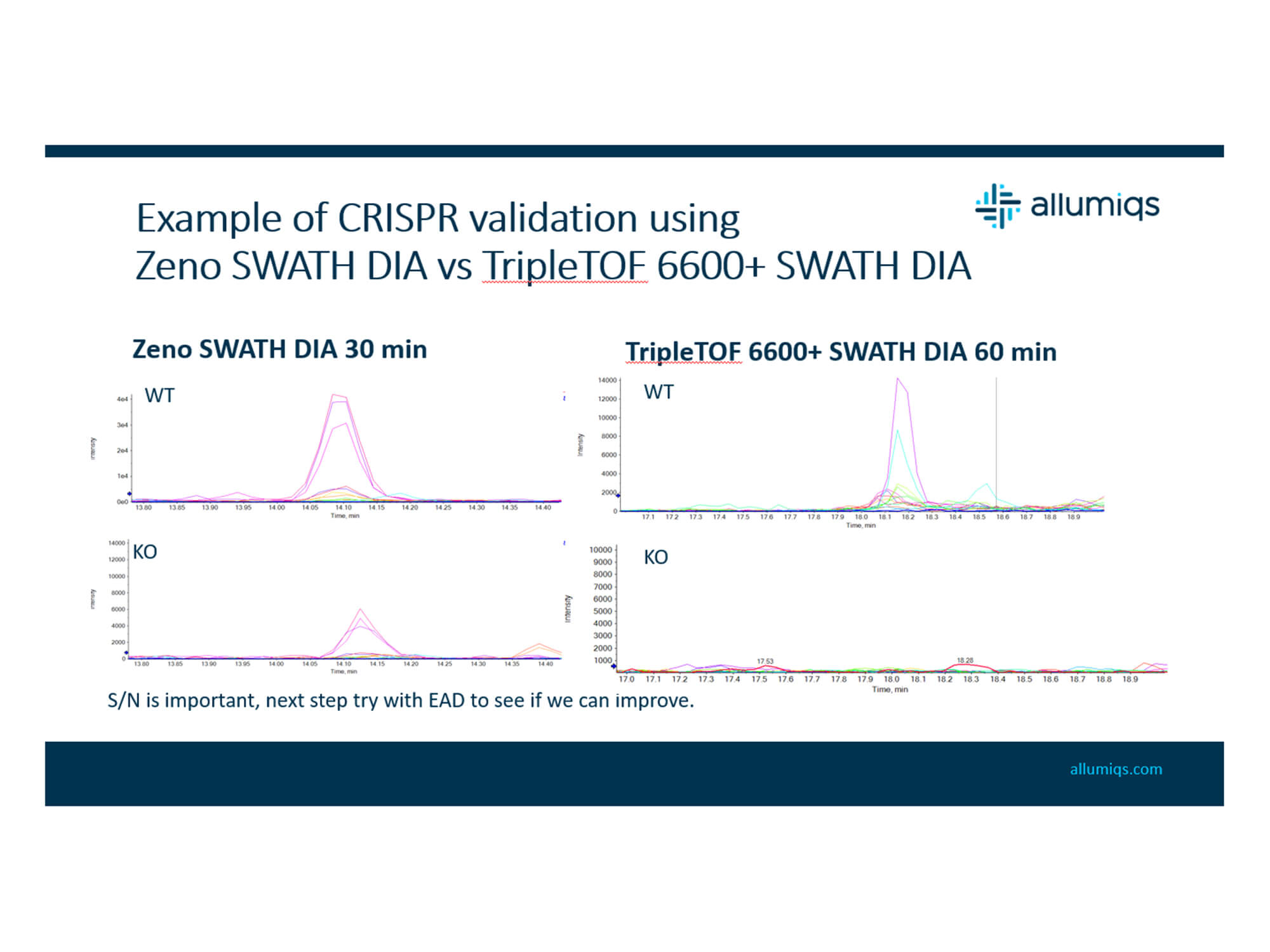
Watch Hugo Gagnon’s extraordinary science
About the presenter

Hugo Gagnon, PhD
Chief Scientific Officer
Allumiqs
Hugo Gagnon is the Chief Scientific Officer of Allumiqs. Previously, he served as the CEO and co-founder of PhenoSwitch Bioscience, which was acquired by Proteoform Scientific to create Allumiqs in 2022.
During his PhD, Hugo used mass spectrometry through different collaborations and core facility services to answer biological questions. He quickly learned that access to mass spectrometry necessitates a considerable resource investment to obtain desirable results. Thus, PhenoSwitch Bioscience was founded with a team of expert scientists to address this need. Hugo studied at Université de Sherbrooke, where he earned a BSc in biochemistry, MSc in pharmacology and a PhD in biochemistry. Hugo has been the driving force behind the innovative LC-MS/MS services offerings tailored with deep data analytics insights and the high-quality reputation the company has developed within the biotech, omics, and mass spectrometry industries. Through the years, Hugo has developed deep expertise in LC-MS/MS-based omics, protein science and data science for omics analysis.
Related publications
The fundamentals behind extraordinary science
Technical notes
-
Quantifying 1000 protein groups per minute of microflow gradient using Zeno SWATH DIA on the ZenoTOF 7600 system
-
Zeno SWATH data-independent acquisition (DIA) applied to high-throughput proteomics analysis of human plasma
-
Zeno MS/MS with microflow chromatography powers the Zeno SWATH data-independent analysis (DIA) workflow to quantify more proteins