The Industry’s Favorite Protein Assay
Capillary electrophoresis sodium dodecyl sulfate (CE-SDS) is the only way to obtain automated, quantitative purity data (i.e. glycosylated vs non-glycosylated) on both intact and reduced monoclonal antibodies. It is quantitative, automated and fast.
CE-SDS data acquired by some technologies is regularly manipulated and enhanced. This may mask weaknesses in hardware and chemistries, and more importantly data quality. Only raw, unprocessed data can provide the confidence critical to both you and the regulatory for product submission and commercialization.
Only SCIEX CE technology uses circulating liquid temperature control to maximize data quality preventing the need for any data enhancement and manipulation. Additionally, our technology supports a wider range of method options.
The Best Technology Provides the Best Impurity Detection
In addition to quantitative data, automation, and full method development flexibility, the following benefits of SCIEX CE-SDS technology provide the critical confidence that you seek in your purity analysis from analytical development to QC.

Trusted Quantitative Data and Advanced Technology
The SCIEX CE-SDS proprietary replaceable gel technology[1], [2], is referenced in USP chapter <129> [3] and the application is recommended by a published intercompany collaboration.[4]

Compliant Raw Data[5]
Patented circulating liquid cooling of the capillary[6] provides reproducible data that requires no enhancements nor manipulations. SCIEX CE-SDS always gives you secure raw data that better quantitates low MW species.[7]

Sensitive Impurity Detection and Quantitation
Modular PDA or laser-induced fluorescence detection provides at least three orders of magnitude of impurity detection – 0.1% and 0.01% respectively.
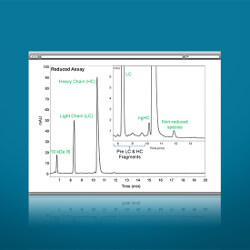
Short CE-SDS Separation Time
The fastest separation without sacrificing resolution (12 min reduced, 18 min. non-reduced).
Flexible Configurations Maximize the Way You Work
SCIEX CE-SDS offers detection with PDA or LIF and two validated chemistry kits.
- The SDS Kit includes components allowing protein sizing
- The IgG purity kit includes control standard specific for IgG characterization
- LIF detection provides for increased sensitivity over standard PDA detection

IgG Purity / Heterogeneity Assay
Part # A10663 Component Quantity/Amount. Capillary, 50 μm I.D. bare-fused silica 2. SDS-MW Gel Buffer - proprietary formulation, pH 8, 0.2% SDS 140 mL. SDS-MW Sample Buffer - 100 mM Tris-HCl, pH 9.0, 1% SDS 50 mL. IgG Control Standard 1 mL. Internal Standard, 10 kDa protein, 5 mg/mL 0.4 mL. Acidic Wash Solution, 0.1 N HCl 100 mL. Basic Wash Solution, 0.1 N NaOH 100 mL.

SDS-MW Analysis Assay
Part # 390953. Component Quantity/Amount. Capillary, 50 μm I.D. bare-fused silica 2. SDS-MW Gel Buffer - proprietary formulation, pH 8, 0.2% SDS 140 mL. SDS-MW Sample Buffer - 100 mM Tris-HCl pH 9.0, 1%SDS 50 mL SDS-MW Size Standard (10 to 225 kDa, 16 mg/mL) 100 μL. Internal Standard, 10 kDa, protein 5mg/mL 0.4 mL. Acidic Wash Solution (0.1 N HCl) 100 mL. Basic Wash Solution (0.1N NaOH) 100 mL.

LIF Upgrade
For ultimate sensitivity in impurity detection, upgrade to Laser Induced Fluorescence (LIF) detection. This upgrade (part # A59494) requires a PA 800 Plus or CESI 8000 Plus. This package consists of a durable solid-state laser and detector that can easily be switched with the PDA detection set-up.

PA 800 Plus Pharmaceutical Analysis System
The PA 800 Plus Pharmaceutical Analysis System is a robust analytical platform that provides characterization of product purity, charge heterogeneity and glycan analysis, to help you with the development and quality control of therapeutic proteins.

Common EZ-CE Cartridge
No need to inventory multiple cartridge types. One EZ-CE cartridge (part # A55625) for Purity, Rapid Charge Variant Analysis, and Fast Glycan Analysis
| Learning Center | |
|---|---|
| Technical Note: Assay of IgG Purity and Heterogeneity Using High-Resolution Sodium Doedecyl Sulfate Capillary Gel Electrophoresis | Download |
| Technical Note: Automation of CE-SDS Sample Preparation for PA 800 plus IgG Purity/Heterogeneity Assays Using a Biomek 4000 Automation Workstation | Download |
| Technical Note: Capillary Electrophoresis in Quality Control: PART I: Application for Therapeutic Proteins | Download |
| Technical Note: Capillary Electrophoresis in Quality Control: PART II: CE-SDS: Method Development and Robustness | Download |
| Flyer: Increase Method Robustness with SCIEX Low pH SDS Sample Buffer | Download |
[1]. Gel US Patent # US7,381,317. Automating the process of gel replacement is disclosed in US.
[2]. Patent # USRE37,606.
[3]. Chemistries referenced in USP Chapter <129>.
[4]. A Series of Collaborations between Various Pharmaceutical Companies and Regulatory Authorities Concerning the Analysis of Biomolecules Using Capillary Electrophoresis.” Chromatographia 2006, 64, September (No. 5/6).
[5]. The PA 800 Plus ensures requirements of 21CFR211.194 can be met by providing raw, unmanipulated data.
[6]. US Patent 5,198,091.
[7]. USP Chapter <129> Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies.