Abstract
In this technical note, the method development strategies to study Enhertu (fam-trastuzumab deruxtecan-nxki) were explored by two analytical technologies, capillary isoelectric focusing (cIEF), and capillary sodium dodecyl sulfate (CE-SDS). This work demonstrates the utility of a multi-capillary CE system for method development and its broad applicability in the life cycle of various biotherapeutic proteins.
Enhertu is an antibody-drug conjugate (ADC), where trastuzumab is the parent antibody. This molecule, a new generation of Cys-ADC, was approved in 2022 to treat unresectable metastatic breast, stomach, and lung cancers. Because Enhertu is a cysteine site specific ADC, it has a homogeneous DAR and the payload is conjugated with the cysteine residues in the hinge region of the trastuzumab molecule1,2 with a high drug-to-antibody ratio (DAR) 8 (Fig. 1).
Due to Enhertu's structure complexity, it is always critical to monitor the charge variant profiles and assess purity during its development. Therefore, a reliable and straightforward method is essential to ensure product quality.
Key features
- Assess purity and charge heterogeneity on a single analytical platform: BioPhase 8800 offers a single analytical platform to assess purity and perform charge heterogeneity analysis.
- A streamlined CE-SDS assay: Straightforward experiment to compare migration pattern between Enhertu and trastuzumab regarding payload effect
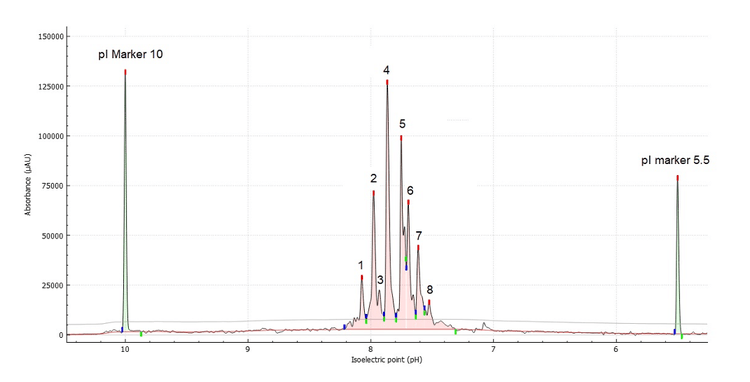
- Screen multiple experimental conditions in a single experiment: Revealing the optimal urea concentration and focusing time to resolve the 8 major charge heterogeneity peaks leveraging the versatility of the multi-capillary environment
Introduction
ADCs are a class of therapeutics consisting of a monoclonal antibody, chemically modified to incorporate a linker bound to a potent cytotoxic drug. ADC technology aims to leverage the specificity and pharmacokinetic properties of the antibody to deliver the cytotoxic drug directly to cancer cells, while minimizing harm to healthy tissue.2
Generally, ADCs have 3 major components: a protein backbone, a cytotoxic payload (or warhead) and a linker that covalently binds the cytotoxic payload to the protein structure. The most popular conjugation chemistry involves binding of the linker through lysine or cysteine groups, resulting in a highly heterogeneous conjugated product. The addition of linker and payload at the cysteine residues at the hinge region adds complexity to structure of ADC challenging well-established chromatography-based platform analytical characterization methods.3
In this work, a single platform approach to screening method conditions for the charge heterogeneity assessment and purity of Enhertu was performed in the multicapillary environment of the BioPhase 8800 system.
Methods
Sample: : Enhertu was obtained from Myonex in a solid form and used without purification. Enhertu was reconstituted to 10 mg/mL in 20 mM Tris-HCl buffer pH 8.
Sample preparation for CE-SDS: Enhertu was analysed under reduced (R) conditions according to the protocol described elsewhere.4
Sample preparation for cIEF: Enhertu was used without any further treatment. The cIEF sample mix was prepared according to the application guide.5 4M urea cIEF gel was used for cIEF of Enhertu and 2M urea cIEF gel for Trastuzumab.
CE-SDS reagents: BioPhase CE-SDS Protein Analysis Kit (PN C30085) was from SCIEX (Framingham, MA). The β-mercaptoethanol (P/N M3148-25ML), and iodoacetamide (P/N A3221) were from Sigma Aldrich (St. Louis, MO, USA).
cIEF reagents: BioPhase Capillary Isoelectric Focusing (cIEF) kit for the BioPhase 8800 system (PN C30101) was from SCIEX (Framingham, MA). Ampholyte 3-10 (P/N17045601) was from Cytiva (Marlborough, MA).
Capillary cartridges: CE-SDS analysis was performed using a BFS capillary cartridge – 8 x 30 cm (P/N: 5080121) and cIEF analysis was performed using the Neutral capillary cartridge – 8 x 30 cm both from SCIEX (Framingham, MA).
Capillary electrophoresis instrument and consumables: The BioPhase 8800 system (P/N: 5083590F) and a starter kit of 4 sample plates, 4 reagent plates and 8 outlet plates (P/N: 5080311) were from SCIEX. CE-SDS analysis was performed using UV detection with a 220 nm filter. cIEF experiments were carried out using UV detection with a 280 nm filter.
Software: BioPhase 8800 software, version 1.2 e-license was used to create methods and sequences for data acquisition.
Results and discussion
Structure of Enhertu
Enhertu is a drug that contains trastuzumab as its protein backbone. It was designed using a cysteine conjugation strategy with a maleimide precursor. After the disulfide bonds at the hinge region are reduced using tris(2-carboxyethyl)phosphine hydrochloride, the drug linkers are added to the reduced trastuzumab backbone leading to a DAR of 8. (Figure 2).1 Cianferani et al.2, performed an intact mass analysis under denaturing conditions using reversed phase liquid chromatography-mass spectrometry. They found that heavy and light chains were separated in the chromatography because of the absence of interchain disulfide bonds. The data shows light chain (LC) bears 1 drug and the heavy chain (HC) bear 3 drugs. No unconjugated light chain or heavy chain were detected in their experiments.
CE-SDS analysis of Enhertu and trastuzumab
Figure 3A shows the overlay between the CE-SDS separations of trastuzumab (green) and Enhertu (red) aligned to the 10 kDa reference internal marker. Notably, there is a slight shift towards lower migration times for both light chain (LC) and heavy chain (HC) from trastuzumab compared to Enhertu.
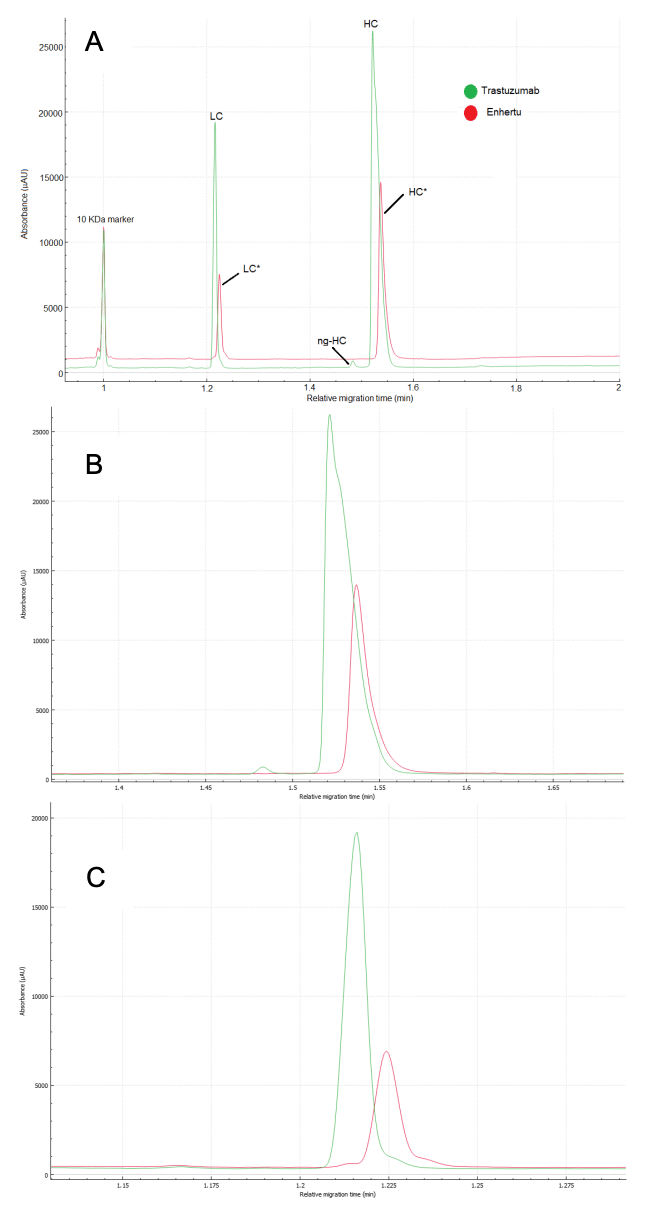
The relative migration time (RMT) difference for LC of trastuzumab and Enhertu is 0.008 min, and for HC, it is 0.016 min. Although these differences are minor in the time domain, the hydrophobic nature of the drug-linker may affect the molecules' movement pattern through the gel. This effect is noticeable from the slightly broader peak shape observed in the conjugated peaks (red traces) shown in Figures 3B and 3C.
Additionally, our results indicate that there is no evidence of unconjugated HC (Fig. 3B), which supports Cianferani's findings. However, Figure 3C shows a minor peak in front of the light chain in Enhertu (red trace), matching the position of the unconjugated light chain, which contradicts Cianferani's rp-LC-MS data.
Generally, purity of the monomer and glycan site occupancy are attributes assessed via CE-SDS under non-reducing conditions. However, due to the nature of this ADC, the non-reduced separation showed a profile similar to the reducing condition except that 2 high molecular weight species were detected as shown if figure 4. The double peak observed for the 10 kDa marker and the light chain may possibly indicate different degrees of alkylation.6 However, further experiments are required to validate these claims.
Charge heterogeneity analysis of Enhertu by cIEF
The first step in method development for cIEF is to evaluate the profile of Enhertu and its parent molecule trastuzumab. Figure 5 shows a comparison of the cIEF separation of trastuzumab (orange) and Enhertu (green), using the platform method.4
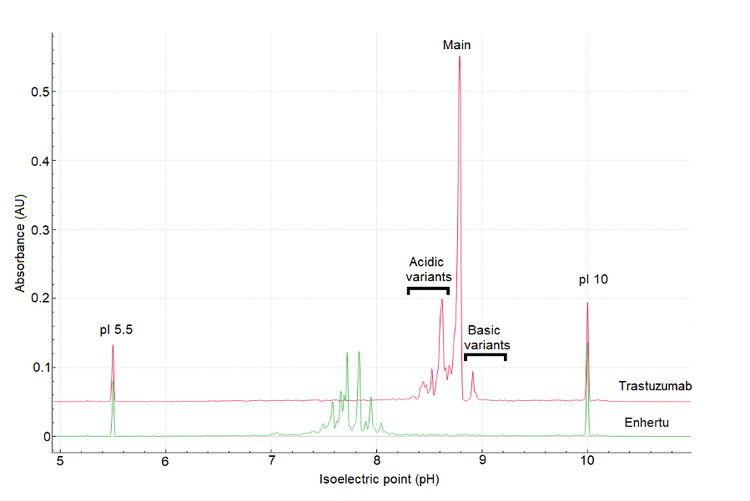
Trastuzumab's profile shows 3 distinct groups: basic, acidic variants, and main peak corresponding to the unconjugated antibody. As expected, Enhertu's profile is more complex and contains considerably more acidic than its parent antibody, trastuzumab.
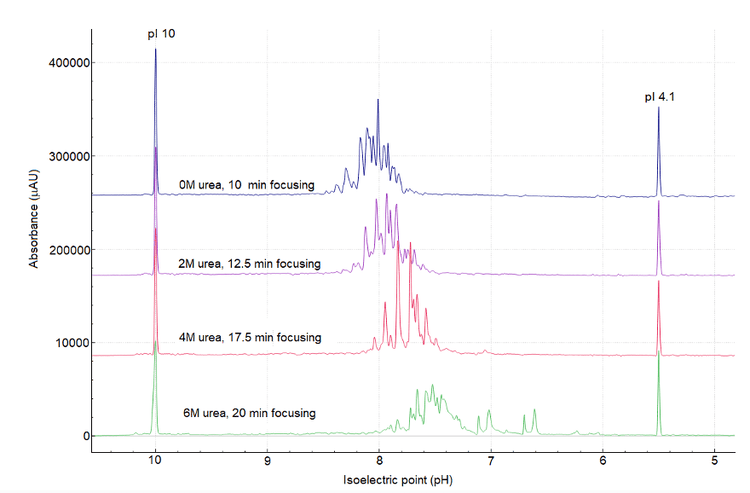
To identify the optimal conditions for cIEF separation and ensure a high-resolution separation profile, urea titration and optimization of separation focusing time were performed. It is well known that urea concentration impacts the focusing time. Generally, higher urea concentration leads to longer focusing times.6 To investigate the impact of urea concentration on the cIEF profile of Enhertu, cIEF separation profiles acquired with various urea concentrations at different focusing times were compared, as depicted in Figure 6.
The profile of Enhertu protein is extraordinarily complex, regardless of the urea concentration, as shown in Figure 6. However, as the denaturing environment of urea increases, the overall isoelectric point of Enhertu shifts slightly to a more acidic region. In the extreme condition of 6M urea, double peaks were observed between pI 7.2 and 6.4, which could be attributed to protein degradation and possibly the loss of payload.
This study establishes that the best cIEF sample mixture conditions to separate Enhertu are 200 µL of 4M urea in cIEF gel; 2 µL of each pI markers 10 and 4.1; 12 µL of broad range 3- 10 ampholyte mix (2% final concentration); 25 µL of cathodic stabilizer, 3 µL of cathodic stabilizer; 10 µL of a 10 mg/mL sample. The separation conditions are 17.5 min of focusing time at 25 kV and 30 minutes of chemical mobilization at 30 kV.
Cianferani et al.2 also successfully analysed the conjugation ratio via intact mass analysis rp-LC/MS under denaturing conditions. The results demonstrated that the payload is conjugated to the inter-bridge disulfhydryl groups leading to a DAR of 8. The light chain contains a DAR of 1 payload, and the heavy chain has a DAR of 3.
Conclusion
- BioPhase 8800 system allowed for the screening of multiple sample preparation conditions in one day, in addition to establishing optimized separation conditions
- CE-SDS experiments revealed differences in migration time and peak shape between Enhertu and the parent antibody trastuzumab due to drug and attached linkers.
References
- Z. Fu, Shijun Li, S. Ha, C. Shi and Y. Zhang; Antibody drug conjugate: the “biological missile” for targeted cancer therapy; Signal Transduction and Targeted Therapy ( 2022) 7:93.
- E. Desligniere, H. Diemer, S. Erb, P. Coliat, X. Pivot, A. Detappe, O. Hernandez-Alba, S. Cianferani. A combination of Native LC-MS approaches for the comprehensive characterization of the antibody-drug conjugate trastuzumab deruxtecan. Front Biosci (Landmark Ed) 2022 Oct 26;27(10):290.
- A. Wakankar, Y. Chen, Y. Gorkan and F. Jacobson; Analytical methods for physicochemical characterization of antibody drug conjugates. mAbs 3:2 (2011) 161-172.
- CE-SDS protein analysis kit for the BioPhase 8800 system. RUO-IDV-05-8662-D.
- cIEF analysis kit for the BioPhase 8800 system; RUO-IDV-05- 8651-C.
- Z. C. Zhua, Y. Chenb, M. S. Ackerman, B. Wang, W. Wub, B. Li, L. Obenauer-Kutner, R. Zhao, L. Taoc, P. M. Ihnat, J. Liub, R. B. Gandhi, B. Qiu; Investigation of monoclonal antibody fragmentation artifacts in non-reducing SDS-PAGE; J. Pharm. And Biomed. Anal 83(2013)89-95.
- S. Mack, I. Cruzado-Park, J. Chapman, C. Ratnayake, G. Vigh; A systematic study in CIEF: defining and optimizing experimental parameters critical to method reproducibility and robustness, Electrophoresis 2009 Dec;30(23):4049-58.
- K. Zheng, Y. Chen, J. Wang, L. Zheng, M. Hutchinson, J. Persson, J. Ji; Characterization of Ring-Opening Reaction of Succinimide Linkers in ADCs; Journal of Pharmaceutical Sciences 108 (2019) 133-141.
- K. Schultz-Kuszak, M. Cui, S. Mack, M. Ostrowski and T. Razunguzwa; Webinar - We all have baggage: A deep dive into the characterization of a complex cIEF electropherogram from an antibody-drug conjugate using a novel integrated icIEF-UV/MS system.
