Development of an ultra-sensitive assay for anti-sense oligonucleotide quantification
Featuring the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready, powered by SCIEX OS Software
Ji Jiang, Eshani Nandita, Lei Xiong
SCIEX, USA
Abstract
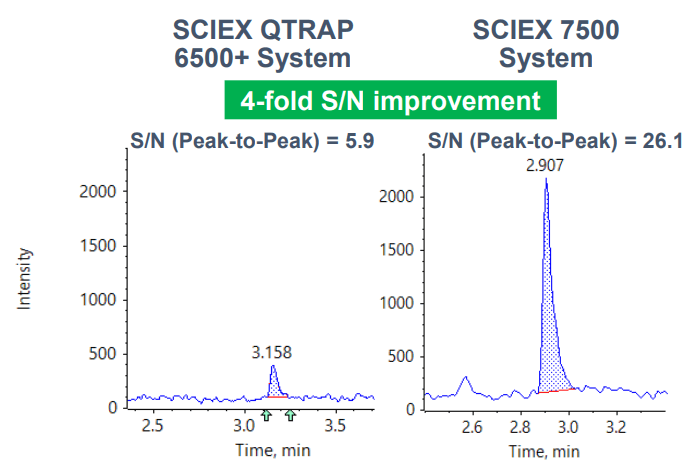
A notable 4-fold improvement in S/N was achieved for the quantification of an anti-sense oligonucleotide (ASO) when comparing the new SCIEX 7500 System with the previous generation of instrumentation (Figure 1). This new level in sensitivity for challenging analytes in matrix opens doors for accurate bioanalysis and metabolism studies with excellent specificity not achievable with orthogonal methods.
Oligonucleotide therapeutics and gene therapies are rapidly gaining attention as their potency improves and delivery challenges are addressed. Modalities such as ASOs are becoming more important due to their high specificity and capability for treating formerly undruggable targets. Recently, an increase in the number of candidates entering clinical trials and approvals have been observed, triggering increased demand for highly sensitive and robust quantitative bioanalytical methods to understand their pharmacokinetic profiles and metabolism. Conventional approaches for oligonucleotide bioanalysis involve ligand-binding assays (LBA) and fluorescence-based detection primarily due to achieving low detection limits. While such techniques can be highly sensitive, there are limitations in coverage of the linear dynamic range (LDR). Moreover, both assays lack specificity in distinguishing oligonucleotide products from related impurities and metabolites. Furthermore, assays utilizing fluorescence-based detection often suffer from low throughput due to extensive run times. Orthogonal technologies such as mass spectrometry (MS) have been routinely employed for bioanalytical studies of oligonucleotides since the platform is capable of both differentiating between target oligonucleotides and associated components: and providing high-throughput capabilities. However, the extent of MS use to date has been limited by its ability to quantify the ultra-low analyte levels in complex biological matrices.
Herein, ASO in extracted human plasma was quantified using liquid chromatography (LC) coupled to a SCIEX 7500 System. The sensitivity of the assay was significantly enhanced over previous generations of instrumentation by multiple hardware improvements on the ion source and the front end of the mass analyzer. The analyte was quantified at 0.2 ng/mL with outstanding reproducibility, accuracy, and linearity, proving the robustness and performance of the developed method. With this method an LC-MS assay addresses what was previously only achievable with LBAs and fluorescence detection, while offering the potential to simultaneously monitor closely related impurities and/or metabolites.
Figure 1. Sensitivity gains for ASO in human plasma. Representative XICs at 0.5 ng/mL are displayed for both systems showing a notable 4-fold increase in sensitivity and S/N.
Key features of anti-sense oligonucleotide quantification using SCIEX 7500 System
- 4-fold S/N improvement was observed compared to the SCIEX QTRAP 6500+ System (Figure 1)
- Lowest limit of quantification (LLOQ) of 0.2 ng/mL was achieved for ASO in human plasma
- Greater ion generation and ion transmission enabling significant gains in sensitivity due to hardware improvements on the SCIEX 7500 System such as the OptiFlow® Pro Ion Source with E Lens™ Technology and the D Jet™ Ion Guide1
- A user-friendly interface providing a single, compliance-ready platform for data acquisition, flexible processing, and management within SCIEX OS Software
 Click to enlarge
Click to enlarge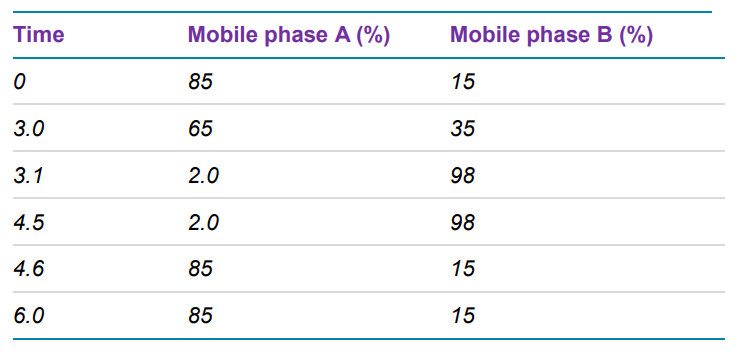 Click to enlarge
Click to enlarge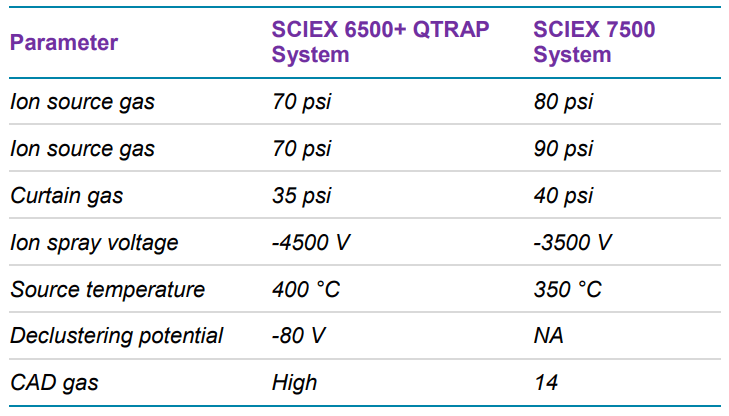 Click to enlarge
Click to enlarge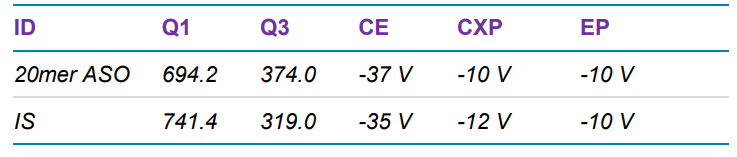 Click to enlarge
Click to enlarge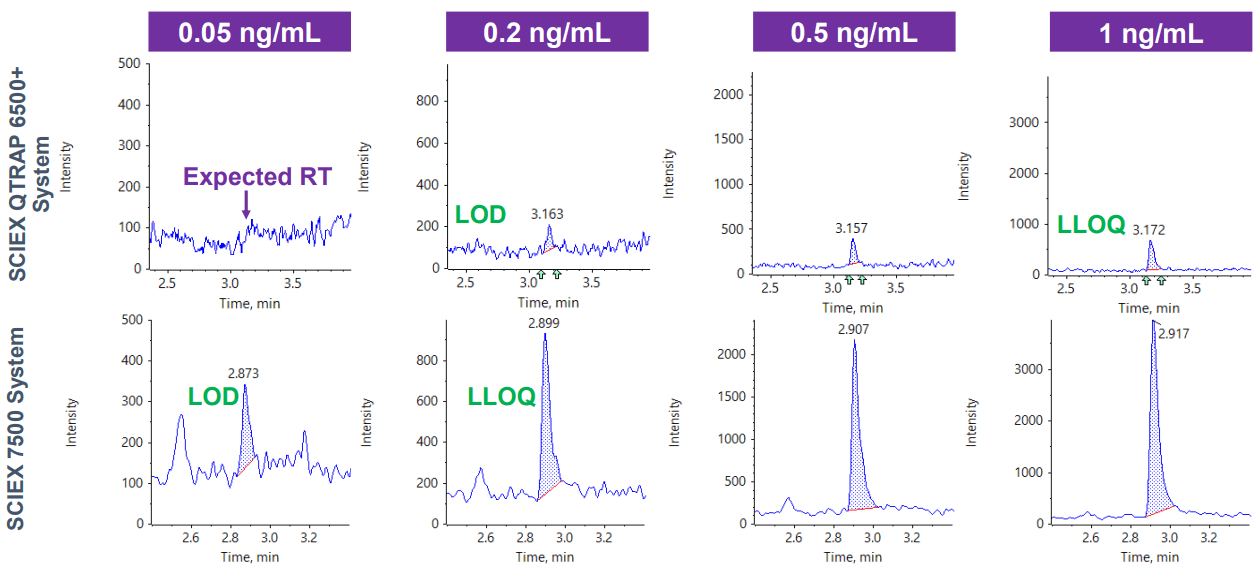 Click to enlarge
Click to enlarge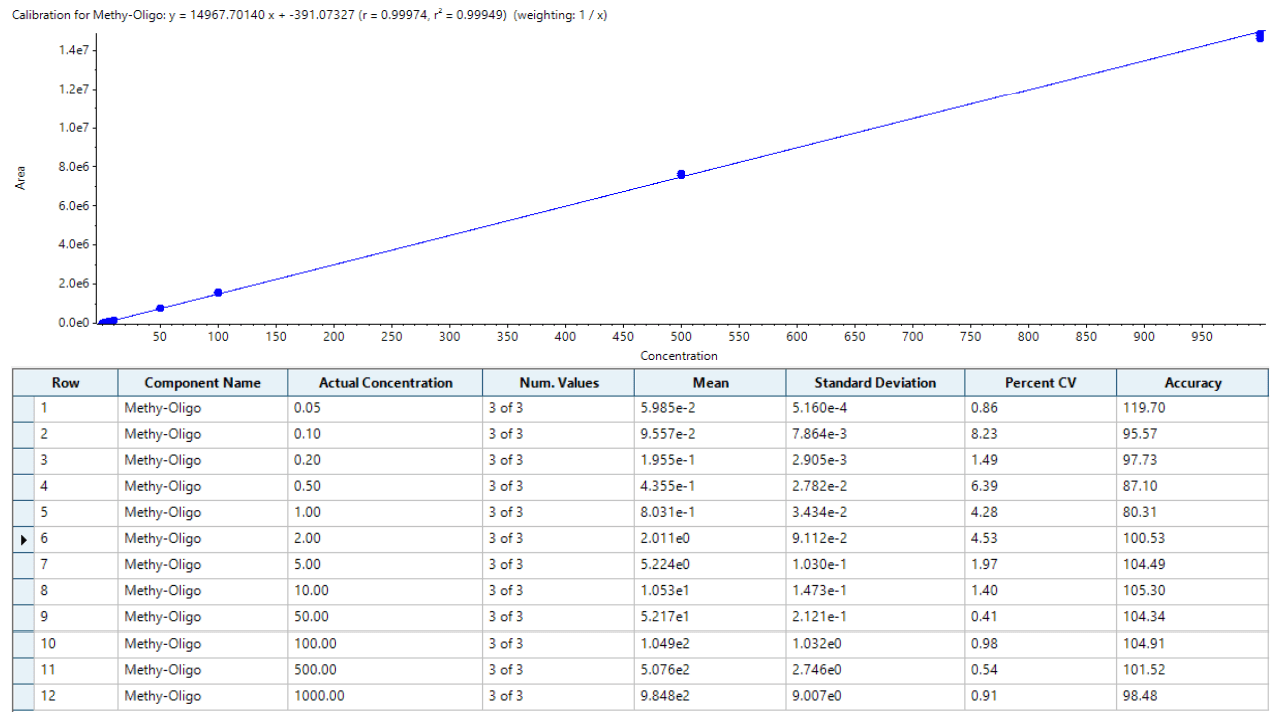 Click to enlarge
Click to enlarge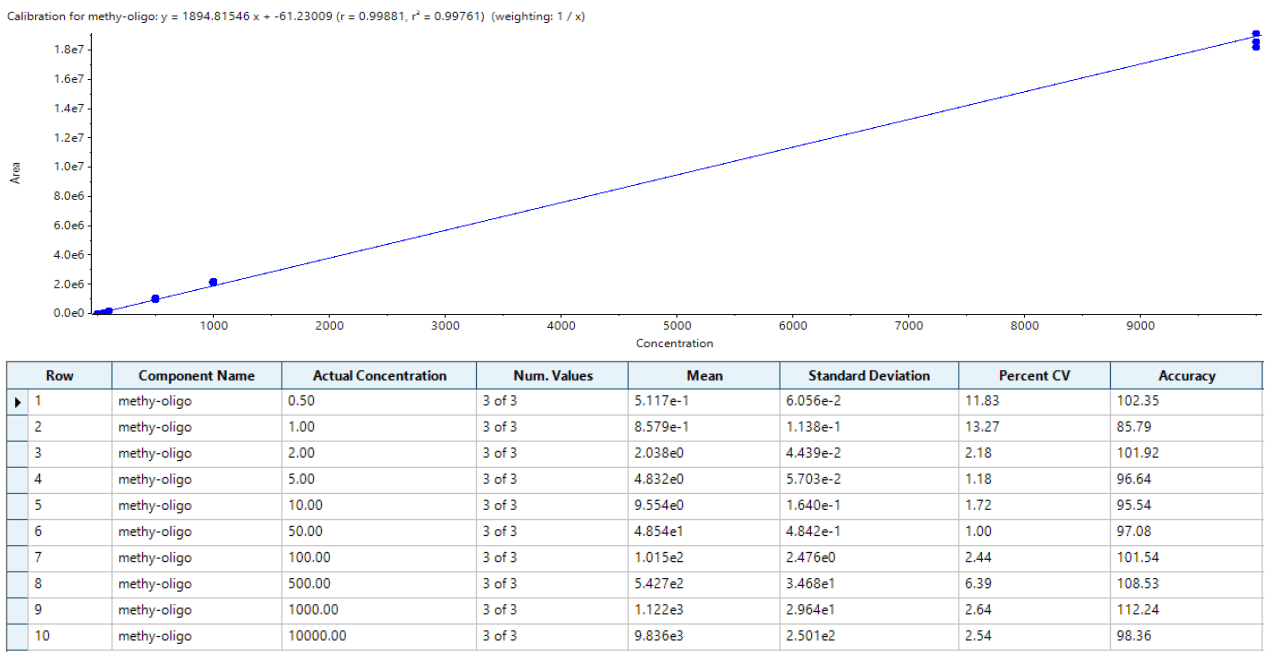 Click to enlarge
Click to enlarge