Low-pg/mL quantitation of cyclic peptides in rat plasma using microflow LC
Achieve low-level quantitation of cyclic peptides using the M5 MicroLC system powered by SCIEX OS software
Eshani Nandita, Ebru Selen and Rahul Baghla
SCIEX, USA
Abstract
This technical note describes the quantitation of cyclic peptides using a microflow trap-and-elute method. Low-pg/mL quantitation was achieved for human atrial natriuretic peptide (ANP) in rat plasma (Figure 1). The linear range observed was between 0.01 ng/mL and 200 ng/mL and the linear dynamic range (LDR) spanned 4.3 orders of magnitude. In addition, the microflow LC method achieved a 5-fold improvement in the lower limit of quantitation (LLOQ) compared to previously published data acquired using analytical flow LC on a SCIEX 7500 system.1 With the microflow trap-and-elute setup, a 50x reduction in solvent consumption was achieved, minimizing waste and providing a more sustainable approach. The M5 MicroLC system and the SCIEX 7500 system were operated using a single platform for method development and data management with SCIEX OS software.

Introduction
Cyclic peptides are polypeptides held in a ring configuration by chemically stable bonds, such as disulfide linkages. For example, the natriuretic peptide (NP) family is a group of genetically distinct cyclic peptides that contain an amino acid ring formed by disulfide bonds. The unique structure of these peptides confers structural stability and conformational rigidity. As a result, cyclic peptides can exhibit enhanced biological activity compared to traditional peptides, making them important therapeutic candidates in cardiovascular diseases.2
With emerging interest in advancing cyclic peptide therapeutics, there is an equivalent drive toward developing robust and sensitive quantitative methods. Current bioanalytical methods lack the sensitivity necessary to quantify cyclic peptides reliably. The sensitivity of LC-MS-based methods is lowered due to the high baseline interference in MS mode and resistance to collision-induced dissociation (CID) in MS/MS mode caused by the tertiary structure of cyclic peptides. The high ion transmission and improved duty cycle of the SCIEX 7500 system enhances sensitivity.
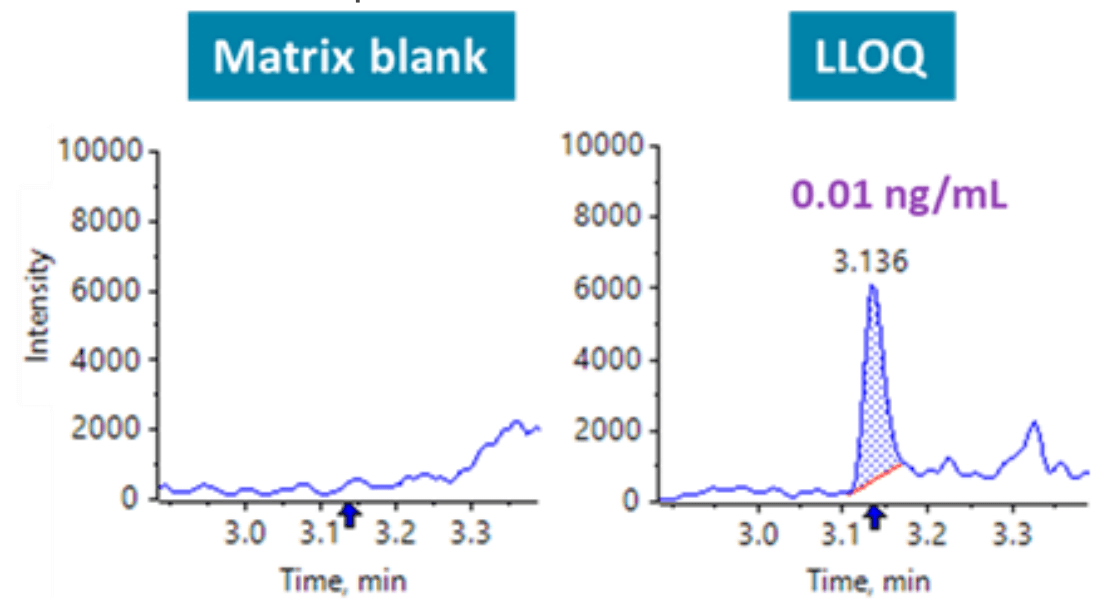
Figure 1. Extracted ion chromatograms (XICs) of a matrix blank and LLOQ sample of human ANP. Matrix interferences were not observed at the retention time of the analyte.
Key features of cyclic peptide quantitation using microflow LC on the SCIEX 7500 system
- Low-level quantitation: Achieve low-level quantitation for human ANP at an LLOQ of 0.01 ng/mL in rat plasma using the M5 MicroLC system and the SCIEX 7500 system
- Robust analytical performance: Achieve accurate quantitative performance with %CV values <12.3% at all concentration levels across an LDR spanning 4.3 orders of magnitude
- Lower solvent consumption: Up to 50x lower solvent consumption was reached using the trap-and-elute workflow compared to the previously published analytical flow method. 1 Reduced LC-MS-grade solvent consumption provides significant cost savings, reduces waste generation and encourages a more sustainable choice.
- Single software control: Easily develop and optimize quantitative workflows using the M5 MicroLC system with seamless integration into SCIEX OS software. Employ a single platform for streamlined method development, data acquisition, processing and management with SCIEX OS software.
Methods
Sample preparation: Rat plasma was protein precipitated and the supernatant was diluted 1:1 with water, which served as the processed biological matrix. Human ANP and a labeled cyclic peptide internal standard (IS) were spiked into the processed rat plasma. The IS concentration was 10 ng/mL. The processed plasma was serially diluted to create the calibration curves for analysis.
Chromatography: The M5 MicroLC system was used for separation in trap-and-elute mode. A 20 µL sample was loaded onto the trap column for analysis. Mobile phase A was 0.1% formic acid in water and mobile phase B was 0.1% formic acid in acetonitrile. The chromatographic conditions used for analyte trapping and separation are summarized in Figures 2A and 2B, respectively. The operating flow rate was set to 50 µL/min for analyte trapping using a Phenomenex Luna C18(2) column (20 x 0.3 mm, 5 µm, 100 Å). The column was used at room temperature.
For analyte separation, the operating flow rate was 5 µL/min using a Phenomenex Kinetex XB-C18 column (50 x 0.3 mm, 2.6 µm, 100 Å). The column temperature was set to 40°C.
Mass spectrometry: Sample analysis was performed using a SCIEX 7500 system in MRM mode. The source was operated in positive ion mode. Collision energy (CE) and other source and MS parameters were optimized to achieve the best sensitivity for MS/MS quantitation. A summary of the source and MS parameters used is shown in Table 1.
Figure 2. LC method settings are embedded in SCIEX OS software. The gradient table for analytical separation (A) and the loading table for the trap column (B). Users can pre-define the flow rate, % mobile phase B and important events, such as “Valve Load” and “Start Gradient 1,” using the LC method settings module.
Table 1. Source and gas parameters.
The MRM transition and method parameters used for human ANP are outlined in Table 2.
Table 2. MRM method parameters.
Data processing: MRM data were processed using the Analytics module in SCIEX OS software using the MQ4 integration algorithm. A weighting of 1/x2 was used for quantitation.
Figure 3. A representative example of the M5 MicroLC system integration with the SCIEX OS software. A microflow LC method can be created and edited using sub-modules of the LC module, including "Run Conditions," "Analytical Separation," "Trap Loading" and "Autosampler." The "Run Conditions" module (A) allows users to view and edit instrument flushing parameters. "Autosampler" module (B) provides parameters related to autosamplers, such as injection and wash.
Figure 4. A single platform for method development, acquisition and data analysis. Easily perform data acquisition using features such as “Batch,” “Queue,” “MS Method,” “LC Method” and “MS Tune.” Evaluate the collected data using the Explorer and Analytics modules of SCIEX OS software.
End-to-end workflow for microflow LCbased analysis
The M5 MicroLC system is integrated with the SCIEX OS software, allowing users to control LC parameters in a single streamlined, user-friendly interface. The software is intuitive and allows for easy method development and optimization of critical parameters. In the LC method module, users can view and control the instrument run conditions (Figure 3A), analytical separation and trapping gradients (Figures 2A and B) and autosampler parameters (Figure 3B). Users can also monitor real-time LC pressure traces for the micro trap and column to aid in diagnostics throughout analysis (data not shown).
SCIEX OS software offers several controls for data acquisition and processing (Figure 4). Batch and Queue modules allow users to run and monitor samples during data acquisition. Acquisition controls further allow users to perform MS tuning, microflow LC and MS method setup. The processing component of SCIEX OS software contains tools for data analysis, including the Explorer and Analytics modules.
Figure 5. Amino acid sequence of human ANP. The cyclic peptide is composed of 28 amino acids and 1 disulfide bridge between cysteine residues.
Cyclic peptide quantitation results
In this workflow, a sensitive LC-MS/MS method was developed to quantify cyclic peptides in rat plasma. Human ANP was used as a model cyclic peptide for this study and is composed of 28 amino acids and 1 disulfide bridge between cysteine residues (Figure 5).
Human ANP was spiked into processed rat plasma at concentrations ranging from 0.01 ng/mL to 200 ng/mL. Concentration levels in the calibration curve were measured in triplicate.
Table 3. Concentration, accuracy and precision for human ANP.
The %CV was expected to be <20% and accuracy was expected to be between 80% and 120% at the LLOQ. At concentrations higher than the LLOQ, the %CV was required to be <15% with accuracy between 85% and 115%.
An LLOQ of 0.01 ng/mL was achieved (Figure 1). No significant matrix interferences were observed at the retention time of the analyte. The implementation of microflow LC resulted in a 5-fold improvement in sensitivity, compared to a prior analytical flow LC-MS/MS method on a SCIEX 7500 system.1
Calculated concentrations for all calibration points were within ±15% of the nominal value with %CV values <12.5%, demonstrating high reproducibility (Table 3).
Linearity was achieved between 0.01 ng/mL and 200 ng/mL with a correlation coefficient (r2 ) >0.99. An LDR spanning 4.3 orders of magnitude was achieved (Figure 6).
Figure 6. Calibration curve for human ANP. The linear range covered 0.01 ng/mL to 200 ng/mL with an LDR spanning 4.3 orders of magnitude.
Conclusion
- Low-level quantitation was achieved for human ANP at an LLOQ of 0.01 ng/mL with accurate quantitative performance and %CV <12.3%
- A seamless integration of the M5 MicroLC system with SCIEX OS software was demonstrated for the quantitation of a cyclic peptide
- Linearity was demonstrated between 0.01 ng/mL and 200 ng/mL, providing an LDR spanning 4.3 orders of magnitude
- Compared to previously published data acquired using analytical flow LC on a SCIEX 7500 system, 1 a 5-fold improvement in LLOQ was achieved using a microflow LC workflow
- Up to 50x lower solvent consumption was achieved with the microflow method using a trap-and-elute setup compared to a previously published analytical flow method.1 Minimizing LC-MS grade solvent consumption provides increased cost savings, reduced waste generation and promotes a more eco-friendly option.
- User-friendly and streamlined integrated data acquisition, processing and reporting were performed using SCIEX OS software as a single platform
References
- Improved LC-MRM quantification sensitivity for cyclic peptides from the natriuretic peptide family. SCIEX technical note, RUO-MKT-02-11883-A.
- Das BB, Solinger R (2009) Cardiovasc. Hematol. Agents Med. Chem. 7(1), 29-42.
 Click to enlarge
Click to enlarge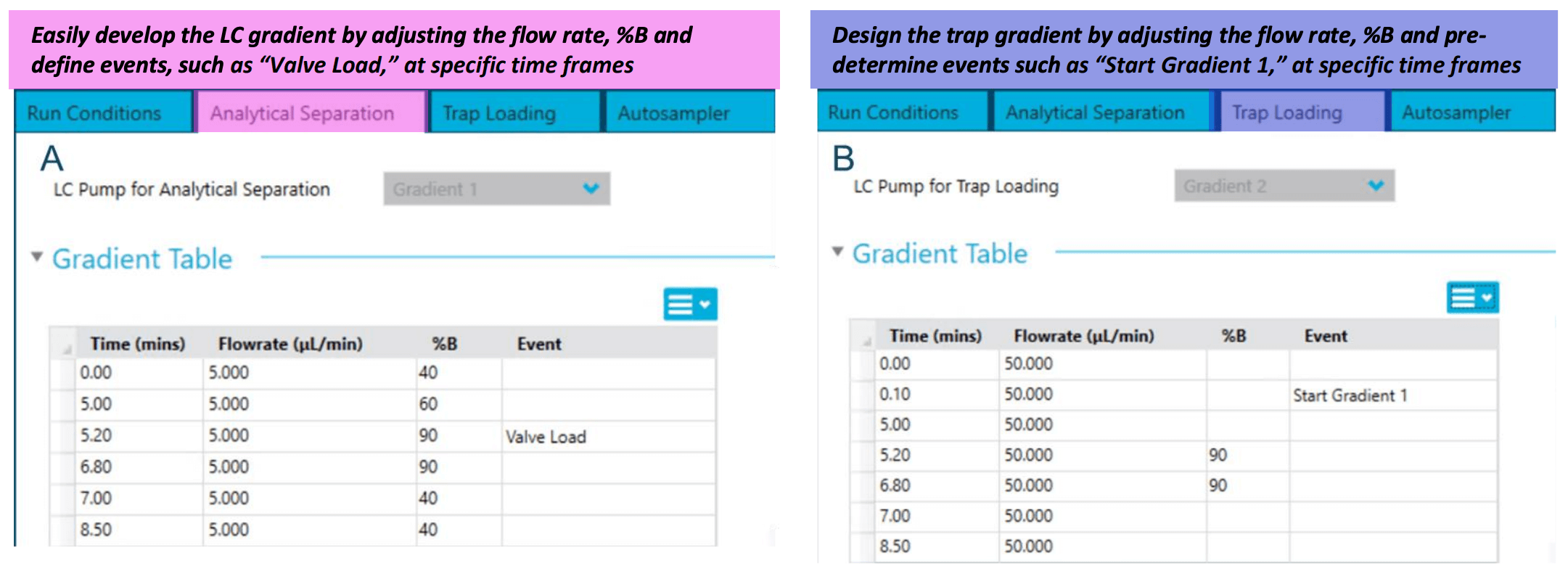 Click to enlarge
Click to enlarge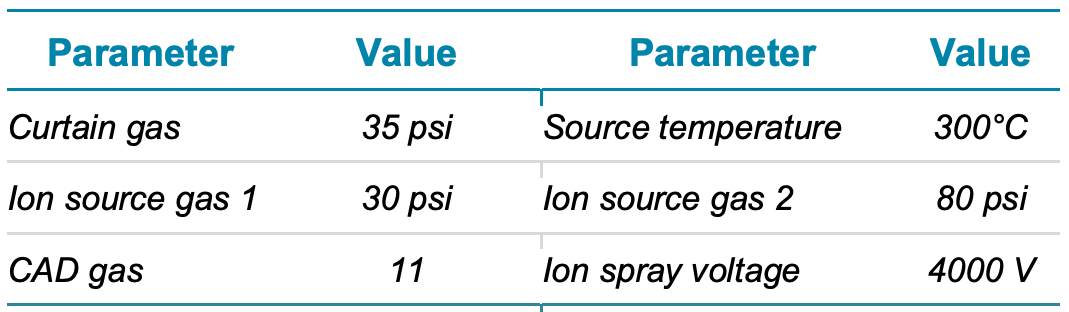 Click to enlarge
Click to enlarge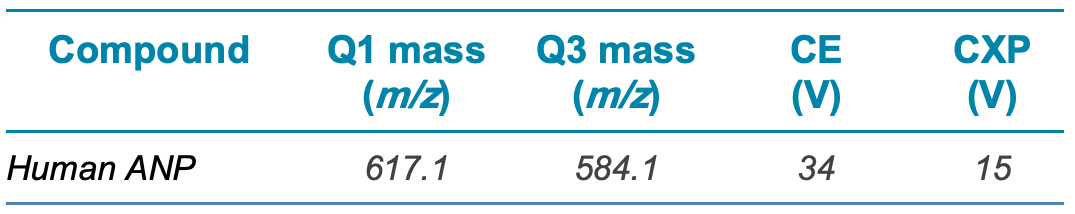 Click to enlarge
Click to enlarge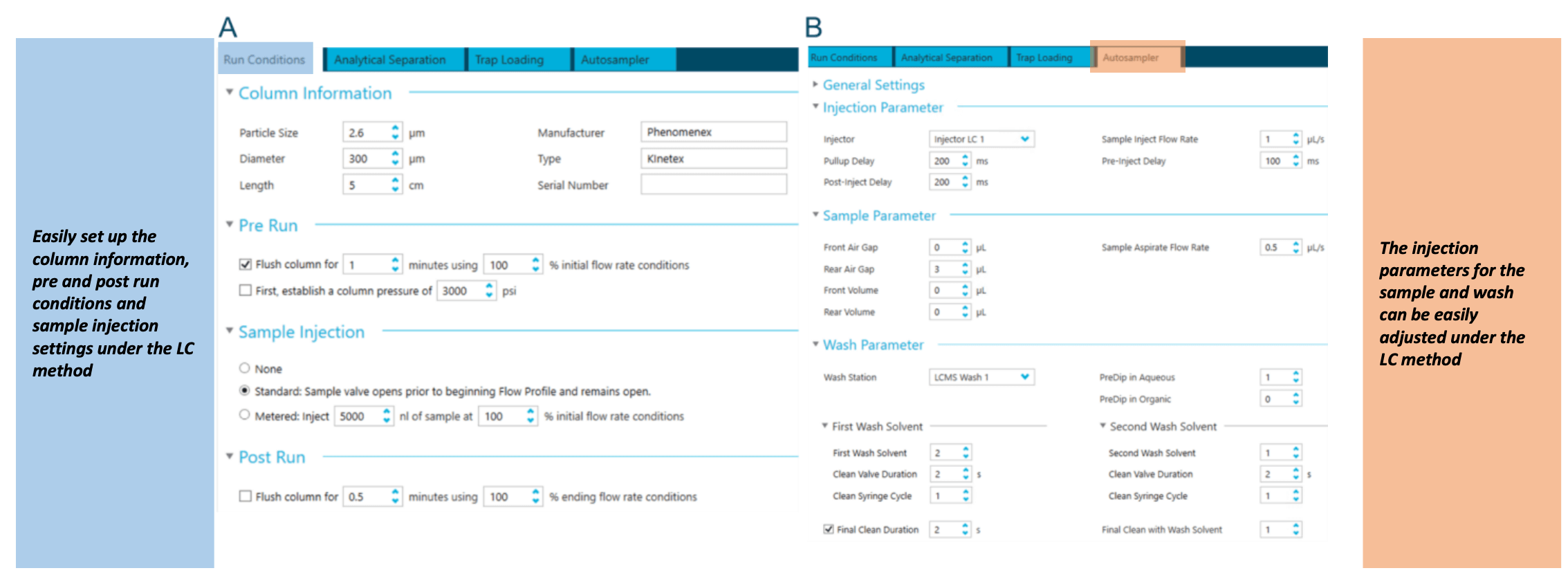 Click to enlarge
Click to enlarge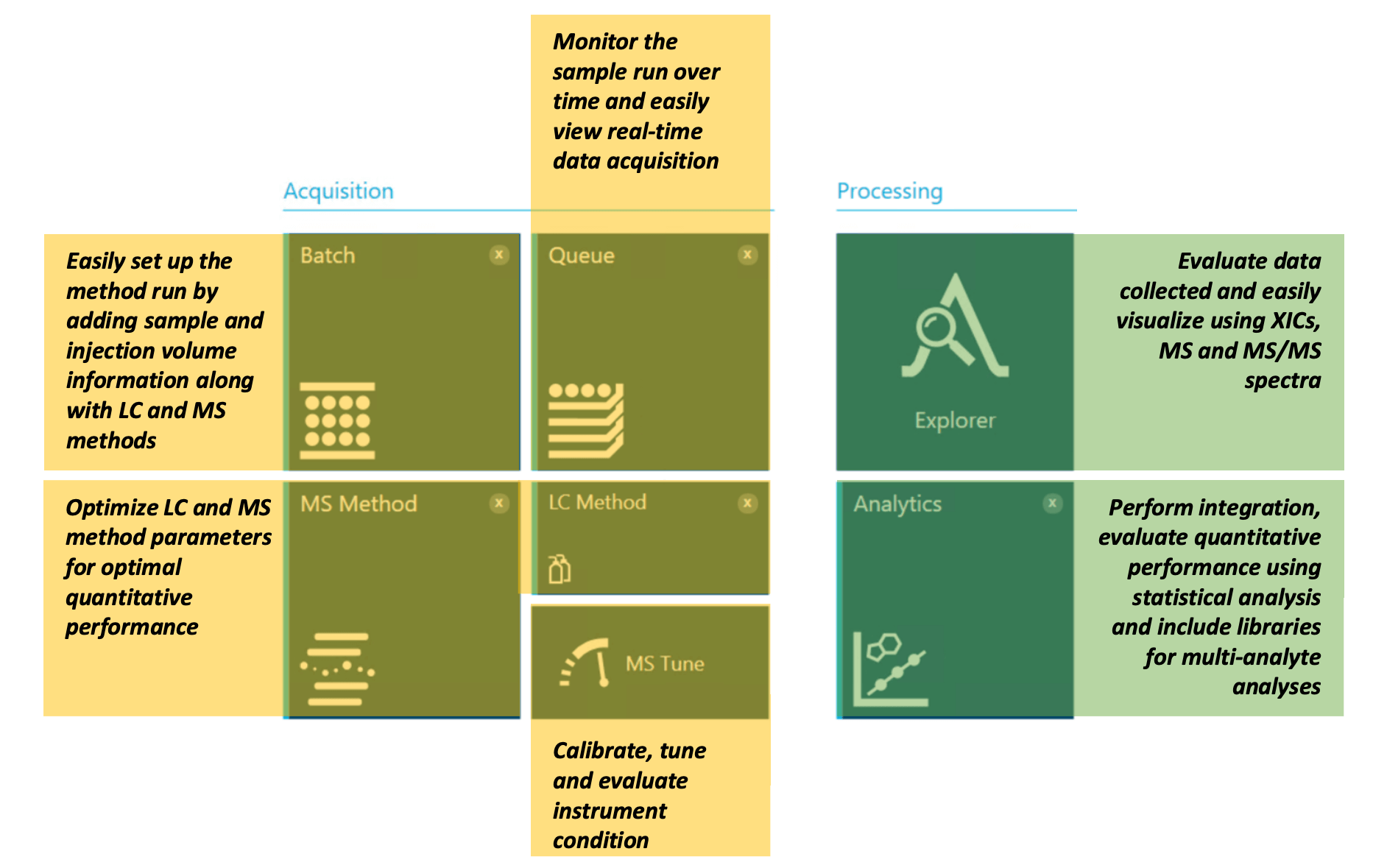 Click to enlarge
Click to enlarge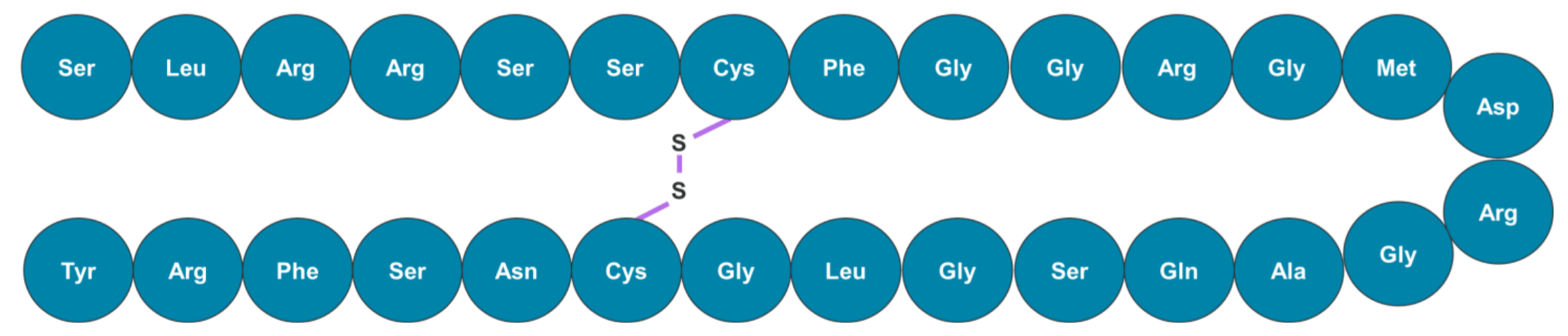 Click to enlarge
Click to enlarge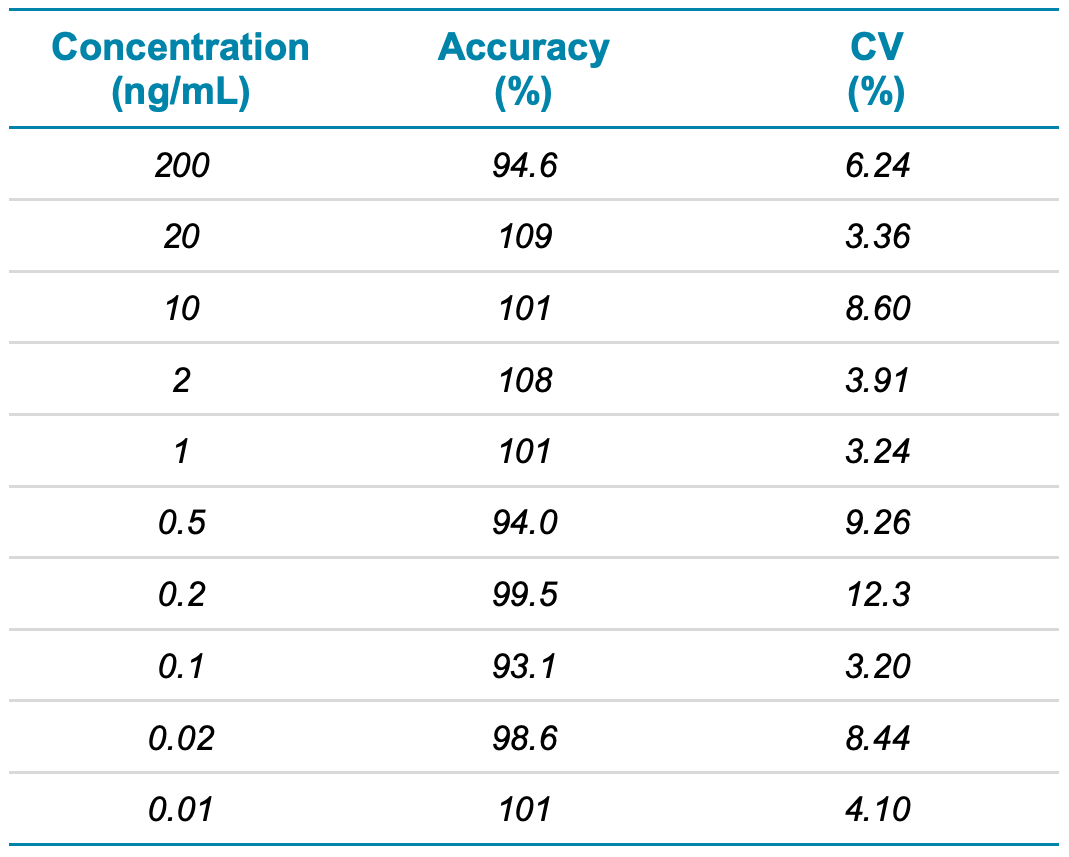 Click to enlarge
Click to enlarge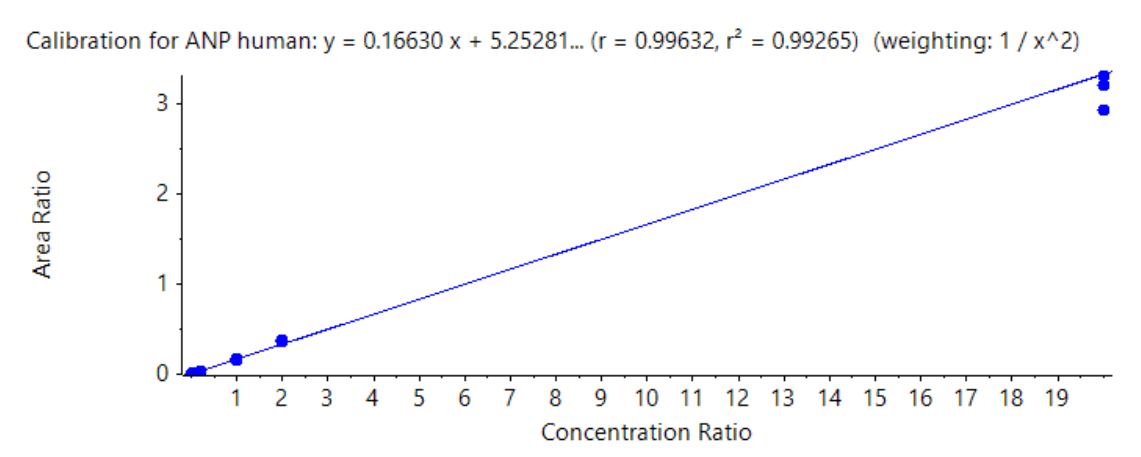 Click to enlarge
Click to enlarge