One-platform strategy for comprehensive characterization of the bispecific antibody, emicizumab, using the BioPhase 8800 system
Zhichang Yang, Marcia Santos, Tingting Li and Sahana Mollah
SCIEX, USA
Introduction
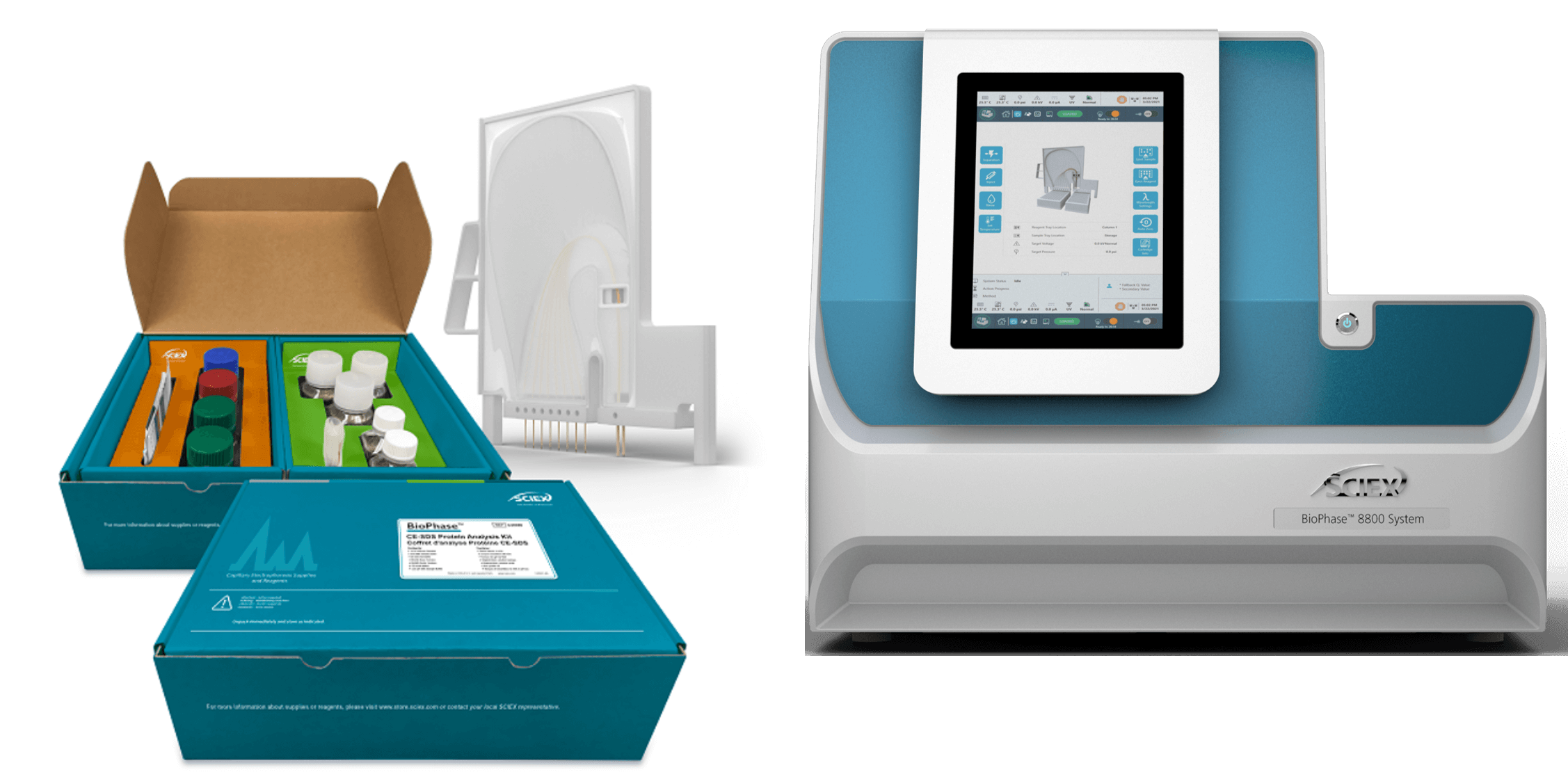
Scientists in the biopharmaceutical industry faced with challenges of manufacturing and characterizing bispecific monoclonal antibodies can now avoid utilizing multiple platforms to monitor drug purity and critical quality attributes (CQAs) by using the multi-capillary BioPhase 8800 system from SCIEX (Figure 1). This is a 1-platform system capable of CE-SDS and cIEF for size and charge variant characterization (Figure 2). The multi-capillary platform on the BioPhase 8800 system significantly shortened the timeline for assay development and permitted exploration of some of the best suitable ampholyte compositions.
Bispecific antibodies (bsAbs) can bind 2 antigen or 2 epitopes from the same antigen. bsAbs are becoming increasingly important for protein biopharmaceuticals, as they represent nearly 20% of the clinical antibody pipeline1 for treatments including immune-oncology, immune-mediated and autoimmune disorders, diabetes, asthma and rheumatoid arthritis.2
Emicizumab is a bispecific monoclonal antibody used for the treatment of hemophilia A. Emicizumab mimics the function of coagulation factor VIII by binding to coagulation factor X and IXa to activate the coagulation cascade to form blood clots.3 To ensure this level of specificity and functionality, it is crucial to monitor the desired structure of bsAbs, including correct chain association, during development and production. It is also necessary to characterize undesired chemical and enzymatic post-translational modifications (PTMs) that can affect drug safety, stability and efficacy.
Key features
- The BioPhase 8800 system offers a 1-platform strategy for comprehensive characterization of size and charge variants of bispecific monoclonal antibodies
- Kitted reagents and consumables and pre-assembled cartridges simplify the operation with minimized operator error
- The 8-capillary system delivers reliable results for both CE-SDS and cIEF analysis with very high repeatability across capillaries and injection replicates and expedites the optimization of parameters that can affect separation performance, such as ampholyte compositions
- The parallel processing capabilities of the BioPhase 8800 system allowed for the screening of suitable ampholyte composition for the analysis of emicizumab in one day
- The repeatability analysis of CE-SDS of emicizumab for corrected peak area of all major species under reduced and non-reduced conditions is well below 1%
- The improved algorithm of the BioPhase 8800 system enables data analysis and peak reporting based on isoelectric point (pI), translating to reduced data processing time and analyst intervention
Figure 1. BioPhase 8800 system with chemistry kit and cartridge.
Figure 2. Size and charge variant characterization using the BioPhase 8800 system. Panel A shows CE-SDS results for reduced (green) and intact (red) emicizumab. Panel B shows cIEF result of emicizumab when isoelectric point (pI) markers 10.0, 9.5, 5.5 and 4.1 were co-injected. X-axis of panel B is calibrated pI values
Here, we present a comprehensive characterization of emicizumab using CE-SDS and cIEF using the multi-capillary BioPhase 8800 system. Capillary electrophoresis (CE) offers high-resolution and robust separation of molecules and is an invaluable technique for the characterization of complex biotherapeutics. CE-SDS can monitor protein size variants caused by artificial fragments resulting from insufficient alkylation.4 cIEF can monitor protein charge variants caused by PTMs that are considered CQAs due to their influence on protein stability and potency.5 With this workflow, CE is leveraged to achieve standard characterization of protein size and charge variants using a 1-platform system with high reproducibility and throughput.
Methods
Chemicals: The BioPhase CE-SDS Protein Analysis Kit (PN: C30085), which included SDS-MW separation gel buffer, 10 kD internal standard, basic wash, acidic wash and sample buffer, and the BioPhase 8800 system cIEF kit (PN: C30101), which consisted of CE grade water, cathodic stabilizer, anodic stabilizer, cIEF anolyte, cIEF catholyte, cIEF chemical mobilizer, cIEF urea, cIEF gel, cIEF neutral capillary conditioning solution and cIEF formamide, were from SCIEX (Framingham, MA, USA). The emicizumab (PN: 50242-0920-01) was from Genentech, Inc. (South San Francisco, CA, USA). The iodoacetamide (PN: I6125-5G) and the 2-mercaptoethanol (PN: M3148-25ML) were from Sigma Aldrich (St. Louis, MO, USA). Wide-range pharmalyte (3-10) (PN: 17045601) was from Cytiva (Marlborough, MA, USA). Narrow-range ampholyte (6-8) (PN: 42906.01) was from SERVA (Heidelberg, Germany).
Materials and instruments: The BioPhase 8800 system (PN: 5083590F), BioPhase BFS capillary cartridge (8 x 30 cm; PN: 5080121), neutral capillary cartridge (8 x 30 cm; PN: 5080119) and sample and reagent plates (PN: 5080311) were from SCIEX (Framingham, MA, USA). The shaker-incubator was model Multi-Therm (Part # H5000-H) from Benchmark Scientific (NJ, USA).
Data acquisition and processing: Data was acquired and processed using the BioPhase software version 1.0.
Sample preparation for CE-SDS: The sample preparation process followed the standard SDS-MW analysis assay kit protocol. Briefly, 4 µL of emicizumab (30 µg/µL) was combined with 91 µL of SDS-MW Sample Buffer, followed by 2 µL of 10 kDa Internal Standard and 5 µL of 250 mM iodoacetamide for non-reduced sample or β-mercaptoethanol (β-ME) for the reduced sample. The sample mixture was vortexed, centrifuged and then heat denatured at 70°C for 10 min. Afterwards, the sample was then cooled to room temperature and 100 µL of sample solution was transferred to the sample plate for CE-SDS analysis.
Sample preparation for cIEF: The 4.0M urea-cIEF gel buffer used for cIEF on the BioPhase 8800 system was prepared by dissolving 2.402 g of urea in 7 mL of cIEF gel buffer. Once dissolved, cIEF gel buffer was added to reach a total volume of 10 mL. The 4.0M urea-cIEF gel buffer was then filtered using a membrane with a 5 μm pore size. The buffer was stored at 2-8°C before use.
Emicizumab at a 5mg/mL concentration was mixed with the reagents listed in Table 1 for BioPhase cIEF analysis. We tested different combinations of wide-range ampholyte (3-10) (W) and narrow-range ampholyte (6-8) (N) to improve the separation resolution of charge variants of emicizumab. The tested ratios included 7:1, 5:1 and 3:1, W/N (v/v). Pure wide-range ampholyte was also tested. Each sample mixture was mixed thoroughly at room temperature and 100 μL aliquots were placed in the injecting well positions of the injection sample plate.
BioPhase SDS-gel electrophoresis: This study was carried out using the BioPhase 8800 system equipped with UV absorbance detection at 220 nm. The separations were accomplished using the BioPhase BFS capillary cartridge filled with SDS-MW separation gel buffer. The capillary conditioning method was used, as shown in Table 2. Capillary conditioning was performed as the first step of each sequence. The operation temperature for both the sample storage compartment and the separation cartridge was set to 25°C.
The separation method was performed as shown in Table 3.
cIEF on the BioPhase 8800 system: This study was carried out using the BioPhase 8800 system equipped with UV absorbance detection at 280 nm. The separations were accomplished using the BioPhase neutral capillary cartridge. The operation temperature for the sample storage compartment was set to 10°C and the temperature for the separation cartridge was set to 20°C. The capillary conditioning and separation methods are shown in Tables 4 and 5, respectively. The cIEF capillary conditioning method was performed as the first run of each sequence.
Results and discussions
Purity analysis of emicizumab: Figure 3 shows the electropherograms of non-reduced (red trace) and reduced (green trace) forms of emicizumab analyzed by CE-SDS. Emicizumab has an asymmetric structure with heterogeneous heavy chains (HC) and a common light chain (LC). The heterogenous HCs were not separated, as indicated by a single peak in the electropherogram. No high molecular weight species (HWS) were detected in either reduced and non-reduced forms. The electropherogram of reduced form shows a low molecular weight species (LWS) that could be the non-glycosylated heavy chain.
Figure 3. CE-SDS separation of emicizumab. Reduced (green) and intact (red) forms are both analyzed by CE-SDS.
The LWS-1 and LWS-2 shown in the electropherogram of the non-reduced form could be the non-glycosylated form of the intact protein and HC:HC:LC (HHL), based on the migration time. Further mass spectrometry analysis is needed to confirm the identification of the LWSs. The non-reducing CE-SDS data revealed the amount of intact form emicizumab (HHLL) is greater than 92%. The ratio between LC to HC obtained is about 2:5. The high-resolution separation achieved by CE-SDS provides a strategy to assess drug purity and integrity. Note that the reduced and non-reduced forms of emicizumab were analyzed in separate plates.
The repeatability of CE-SDS analysis of emicizumab under both reduced and non-reduced forms was achieved by putting 6 100 µL aliquots of emicizumab sample into 6 wells of the sample plate. Five consecutive injections were performed from each well. Figure 4 shows an overlay of the 30 replicates for both reduced (Figure 4-A) and non-reduced (Figure 4-B) forms of emicizumab. Table 6 shows the relative standard deviation (%RSD) for 30 injections regarding migration time (MT) and corrected peak area % (CPA%) for both reduced and non-reduced forms of emicizumab. Our results indicate excellent repeatability in general (< 5% RSD%) including %RSD of less than 2% CPA for the lower abundant species.
Figure 4. Repeatability analysis of emicizumab in both intact and reduced form from 30 replicates. Panel A is the result of reduced form of emicizumab analyzed by CE-SDS. Panel B is the result of non-reduced form of emicizumab analyzed by CE-SDS.
Charge variant analysis of emicizumab: During the manufacturing process, monoclonal antibodies (mAbs) heterogeneity could occur due to enzymatic cleavage and chemical post-translational modifications (PTM).6 Many of these PTMs include deamidation, C-terminal lysine truncation, glycation and sialylation, which can change the surface charge distribution of these species changing the molecule's net charge (isoelectric point).7
Characterization of the charge heterogeneity of mAbs is essential for (CQA) assessment to ensure drug safety, efficacy, and potency.
cIEF can separate charge variants with very close pI values and is applied in quality control (QC) and lot release tests in the biopharmaceutical industry.8 Figure 5-A shows cIEF analysis of emicizumab when pure wide range ampholyte (3-10) was used. The BioPhase 8800 system allows the user to calibrate isoelectric point (pI) with multiple pI markers co-injected with the sample. The system also provides the ability to plot the data using pI on the x-axis, where the calibrated pI values were determined using the pI markers. Six consecutive injections were performed for repeatability assessment (Figure 5-B). cIEF separated the acidic variants, major species and the basic variants of emicizumab. It also provided information on relative abundance and pI values of these charge variants with good repeatability (Table 7), proving the robustness and reliability of the assay, essential for CQA assessment.
Figure 5. cIEF analysis of emicizumab using pure wide range ampholyte. Panel A is the result of cIEF analysis of emicizumab. Panel B shows overlay result of 6 timely consecutive injections
Improving charge variants separation resolution by changing ampholyte compositions: Ampholyte mixture composition forms the pH gradient and affects cIEF separation resolution of the charge variants. The pI of the charge variants of emicizumab lies in the range of 6-7, indicating that a narrow range ampholyte (6-8) can help improve separation resolution. Various combinations of wide range ampholytes (3-10) (W) and narrow range ampholytes (6-8) (N), including W/N of 3/1, 5/1 and 7/1 (v/v) were tested for resolution improvement. The total portion of ampholyte was fixed at 4.8% (v/v). As shown in Figure 6, by increasing the amount of narrow range ampholyte, there is an improvement in the resolution of the separation of the charge variants of emicizumab, but also increases the noise level. The parallel processing capabilities of the BioPhase 8800 system allows for the evaluation of different ampholyte combinations simultaneously, shortening analysis time by increasing the throughput by 8 times. In addition to ampholyte composition, other factors such as, concentration of urea, cathodic and anodic stabilizers and pH and concentration of anolyte, catholyte and chemical mobilizer solutions, could also affect the performance of cIEF separations. Optimizing these factors requires careful design of experiments (DOE) and can take a significant amount of time in a traditional single-capillary system. For example, a full factorial DOE with 4 factors, each with 2 input levels, requires 16 runs for optimization, which can take approximately 18 hours using a single-capillary system. However, it only takes 2 runs using the BioPhase 8800 system to expedite the DOE experiments.
Figure 6. Improving cIEF separation resolution with various combinations of ampholyte.
Conclusion
- Comprehensive characterization of emicizumab CQAs (charge and size variants) were performed using the 8 capillary platform and the BioPhase 8800 system
- The robustness and reliability of the system on the analysis ofemicizumab was less than 1% RSD for migration time/calculated pI and less than 5% RSD of CPA% across multiple replicates
- CE-SDS detected low molecular weight species possibly caused by aglycosylation and fragments while cIEF separated charge variants of emicizumab, likely caused by chemically or enzymatically induced PTMs. Information about pI values and relative abundance of the variants were achieved with high repeatability.
- Taking advantage of the parallel processing capabilities offered by the BioPhase 8800 system, we analyzed the effect of multiple ampholyte combinations on the resolution of cIEF separation simultaneously, reducing analysis time
References
- Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. 2021 Jul;20(7):491-495. https://pubmed.ncbi.nlm.nih.gov/33953368/
- Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608. https://pubmed.ncbi.nlm.nih.gov/31175342/
- Knight T, Callaghan MU. The role of emicizumab, a bispecific factor IXa- and factor X-directed antibody, for the prevention of bleeding episodes in patients with hemophilia A. Ther Adv Hematol. 2018 Oct 10;9(10):319-334. 10.1177/2040620718799997
- Wagner E, Colas O, Chenu S, Goyon A, Murisier A, Cianferani S, François Y, Fekete S, Guillarme D, D'Atri V, Beck A. Determination of size variants by CE-SDS for approved therapeutic antibodies: Key implications of subclasses and light chain specificities. J Pharm Biomed Anal. 2020 May 30; https://europepmc.org/article/med/32113118
- Liu H, Ponniah G, Zhang HM, Nowak C, Neill A, Gonzalez-Lopez N, Patel R, Cheng G, Kita AZ, Andrien B. In vitro and in vivo modifications of recombinant and human IgG antibodies. MAbs. 2014;6(5):1145-54; https://pubmed.ncbi.nlm.nih.gov/25517300
- Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010 Aug;9(8):1716-28. https://pubmed.ncbi.nlm.nih.gov/20103567/
- Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, Kirchmeier M, Corvaïa N, Ionescu R, Beck A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009 Sep 15;392(2):145-54. https://pubmed.ncbi.nlm.nih.gov/19497295
- Cao M, De Mel N, Shannon A, Prophet M, Wang C, Xu W, Niu B, Kim J, Albarghouthi M, Liu D, Meinke E, Lin S, Wang X, Wang J. Charge variants characterization and release assay development for co-formulated antibodies as a combination therapy. MAbs. 2019 Apr;11(3):489-499. https://europepmc.org/article/pmc/6512943
 Click to enlarge
Click to enlarge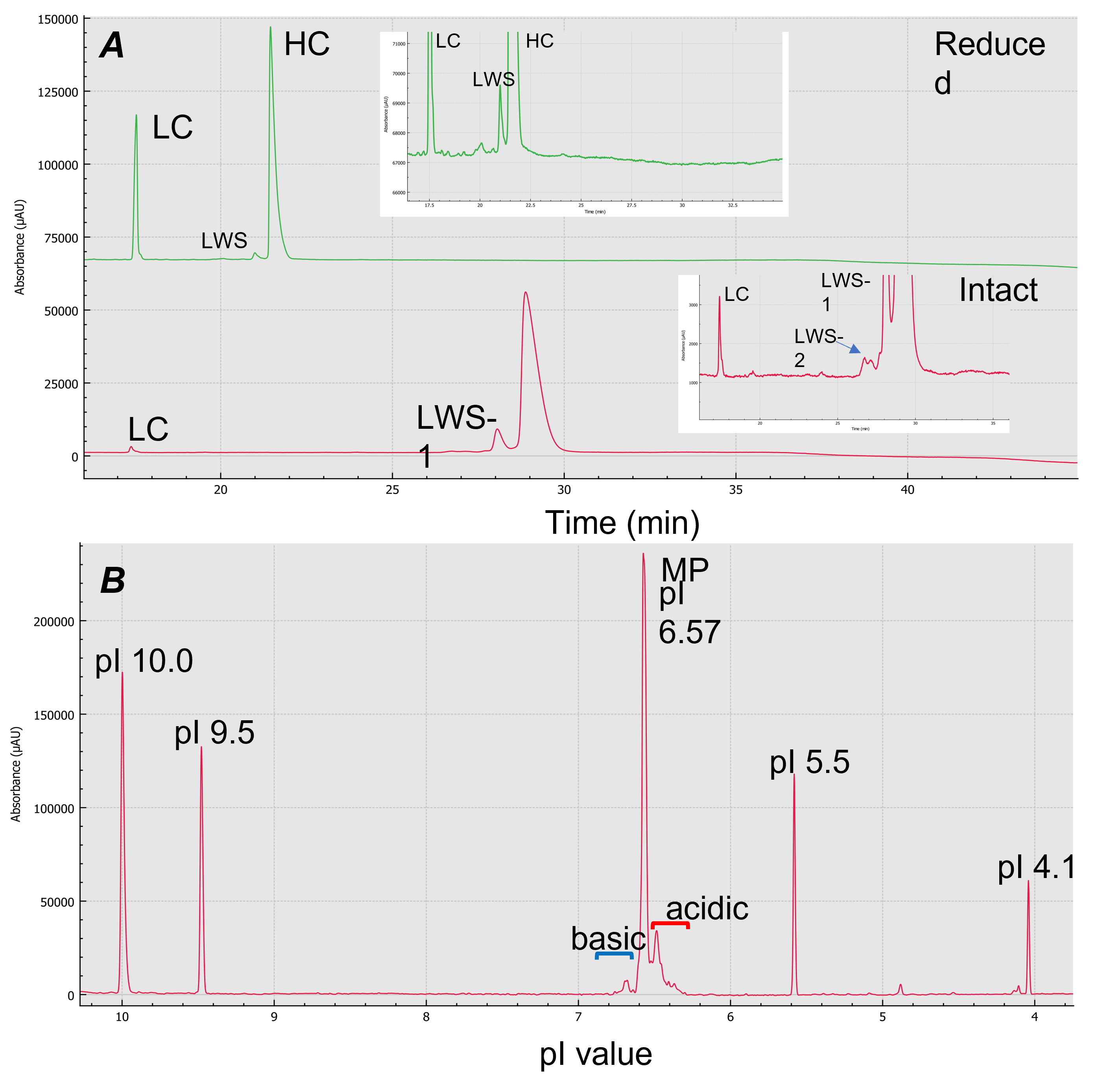 Click to enlarge
Click to enlarge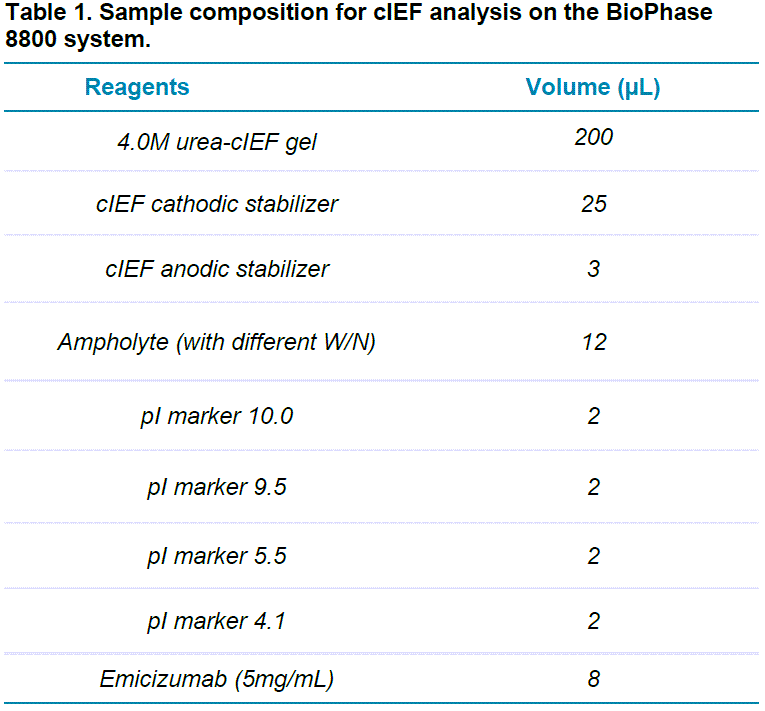 Click to enlarge
Click to enlarge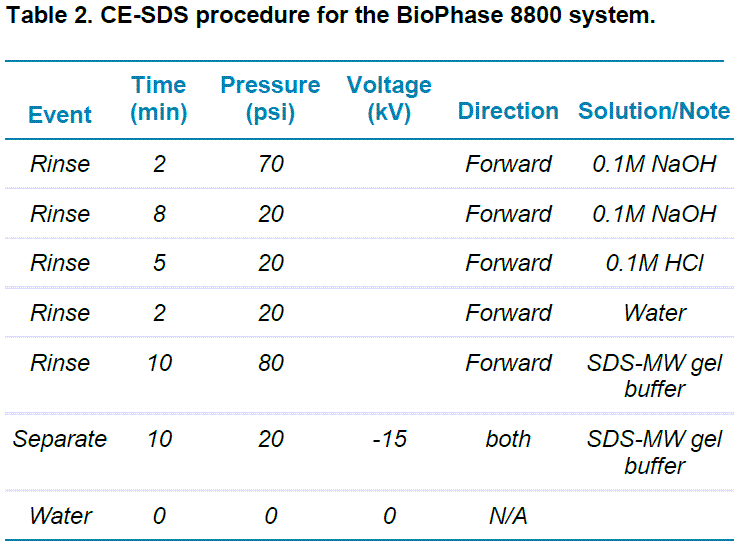 Click to enlarge
Click to enlarge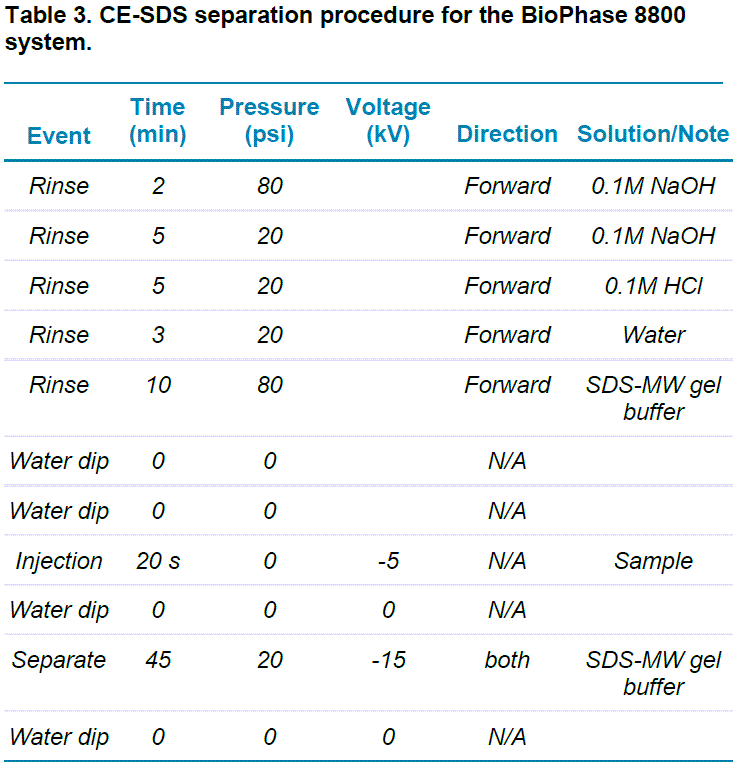 Click to enlarge
Click to enlarge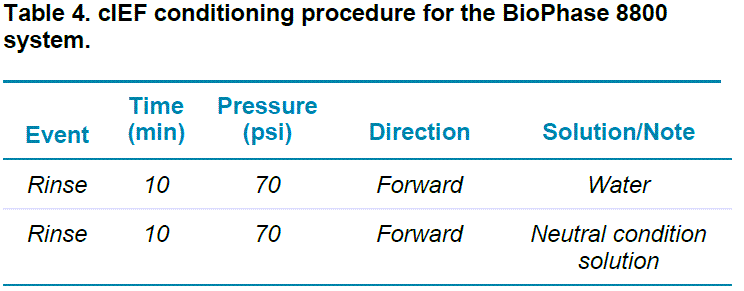 Click to enlarge
Click to enlarge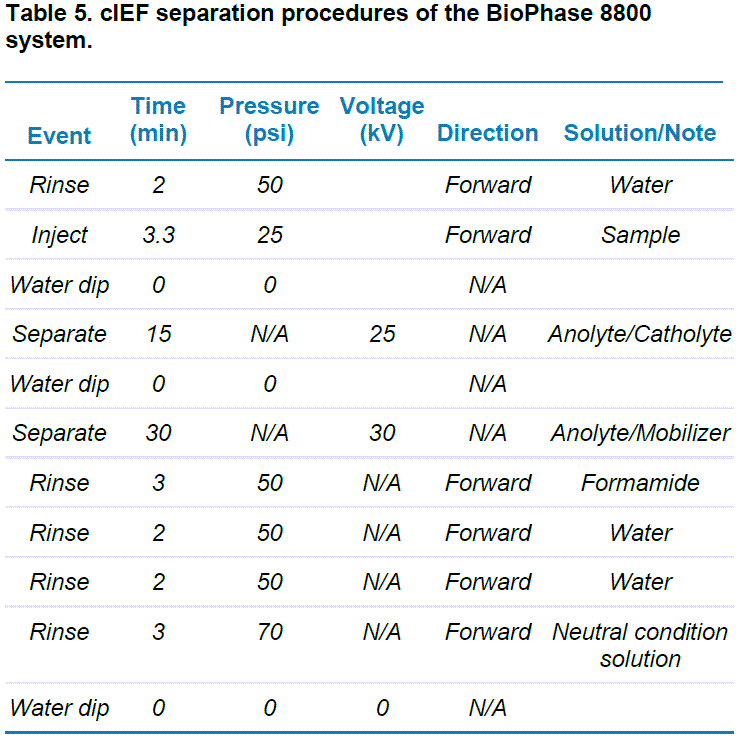 Click to enlarge
Click to enlarge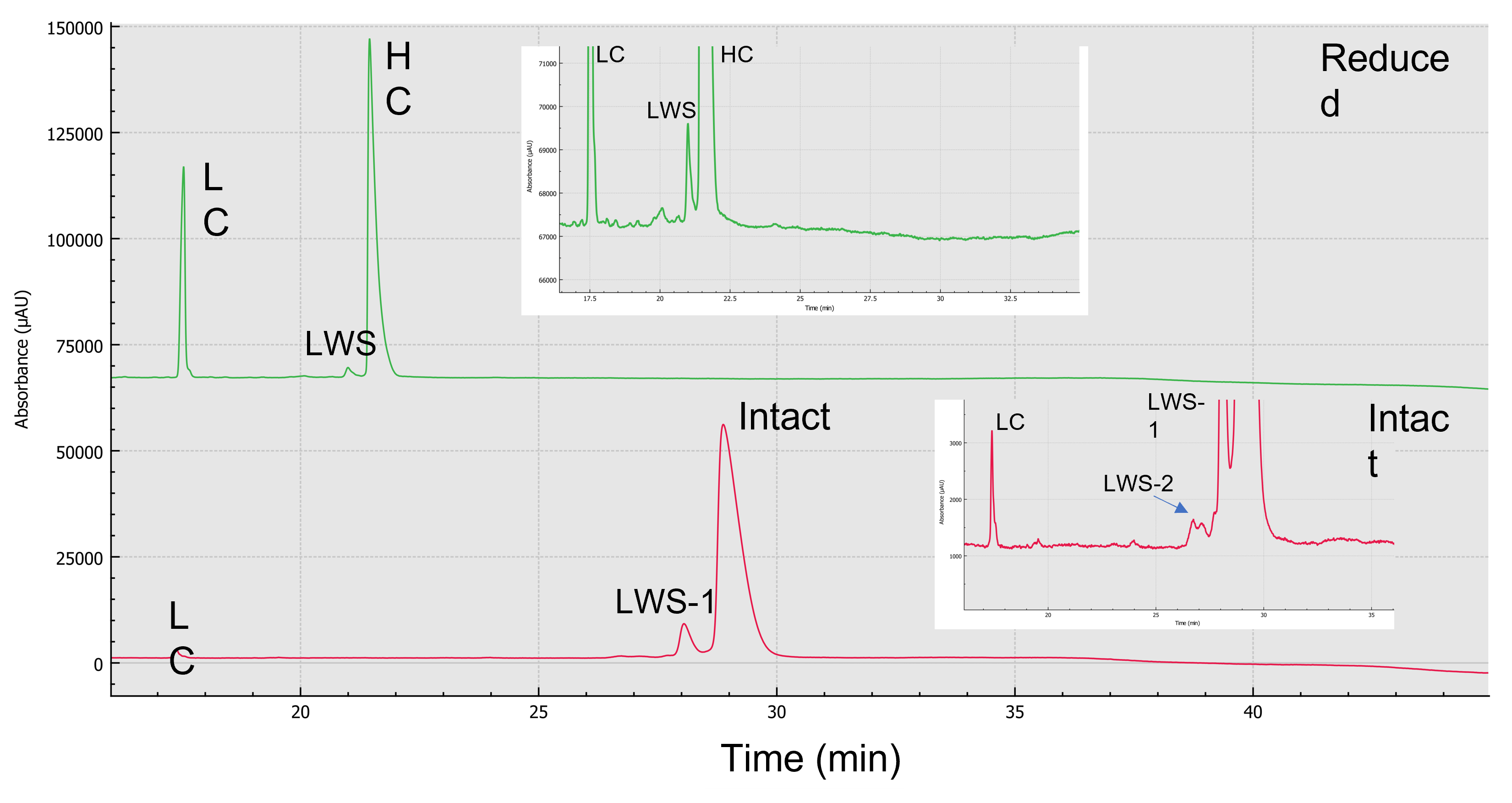 Click to enlarge
Click to enlarge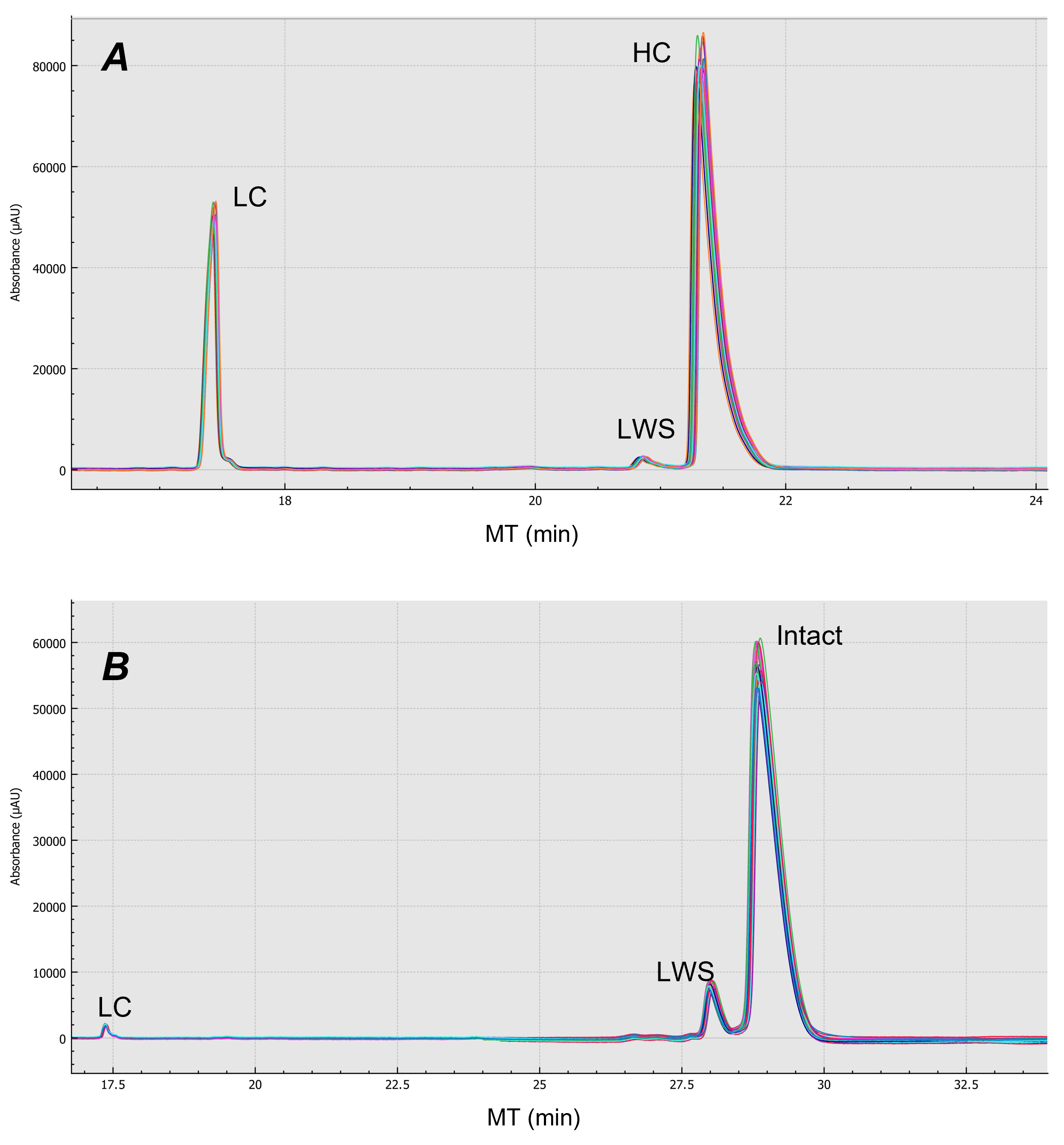 Click to enlarge
Click to enlarge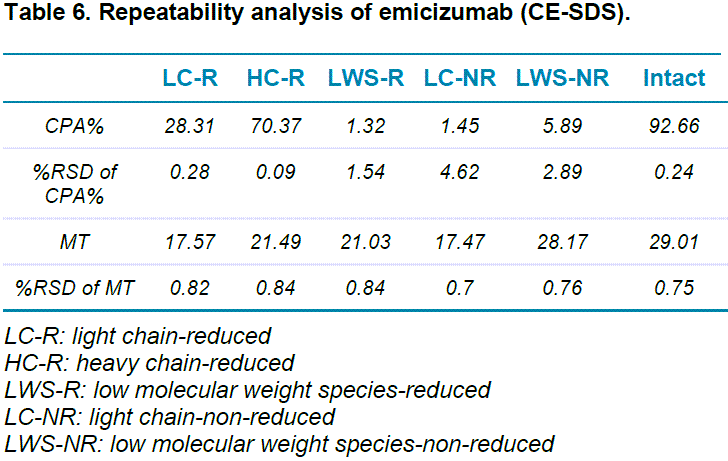 Click to enlarge
Click to enlarge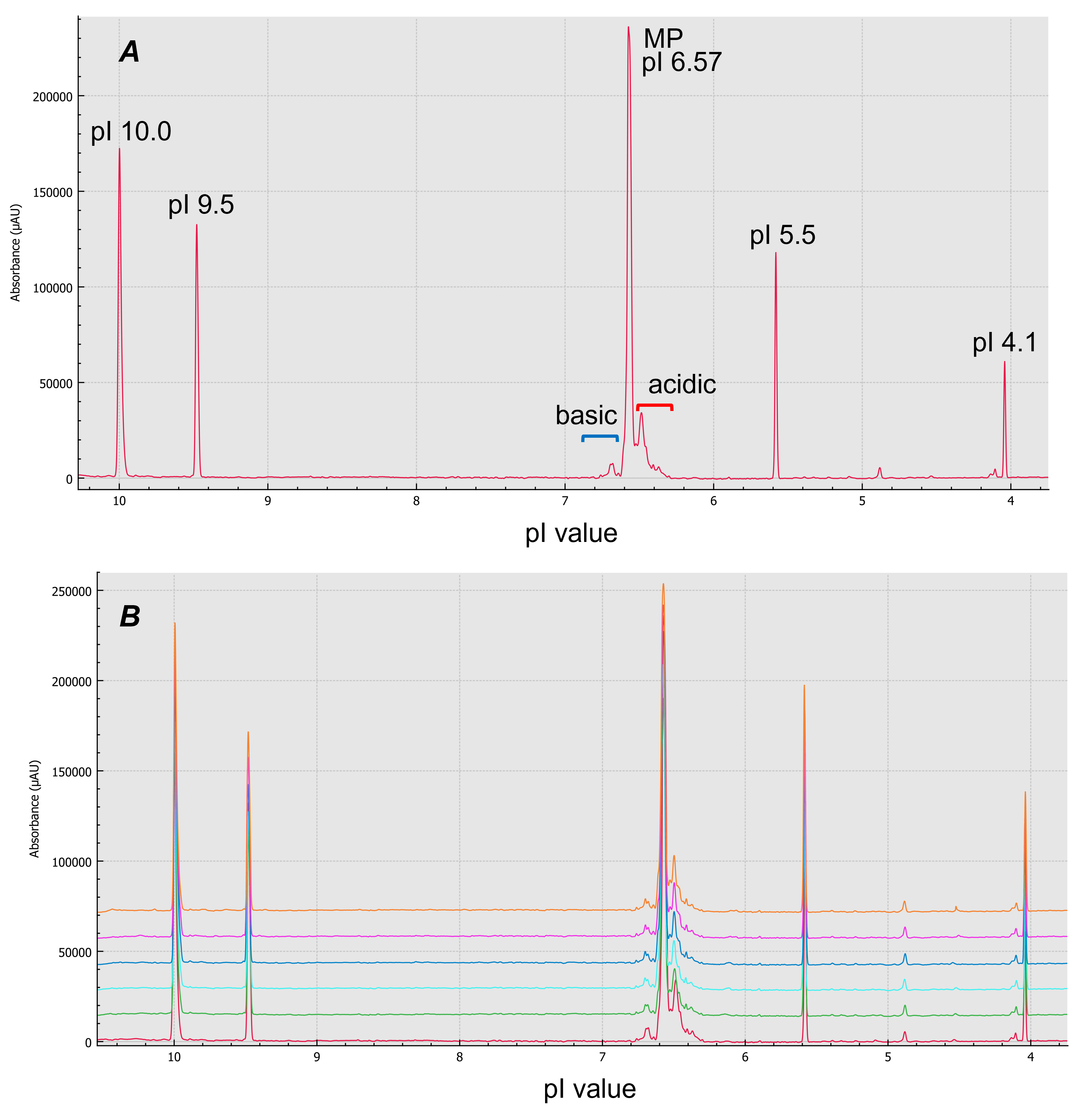 Click to enlarge
Click to enlarge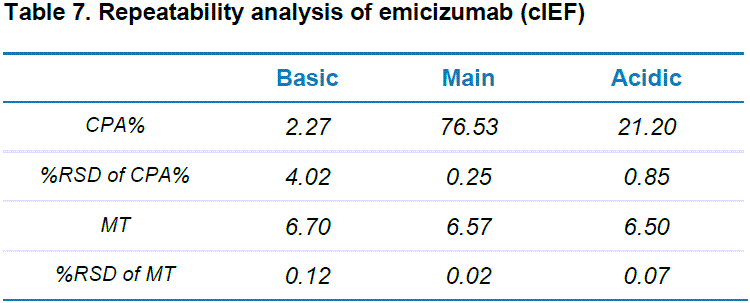 Click to enlarge
Click to enlarge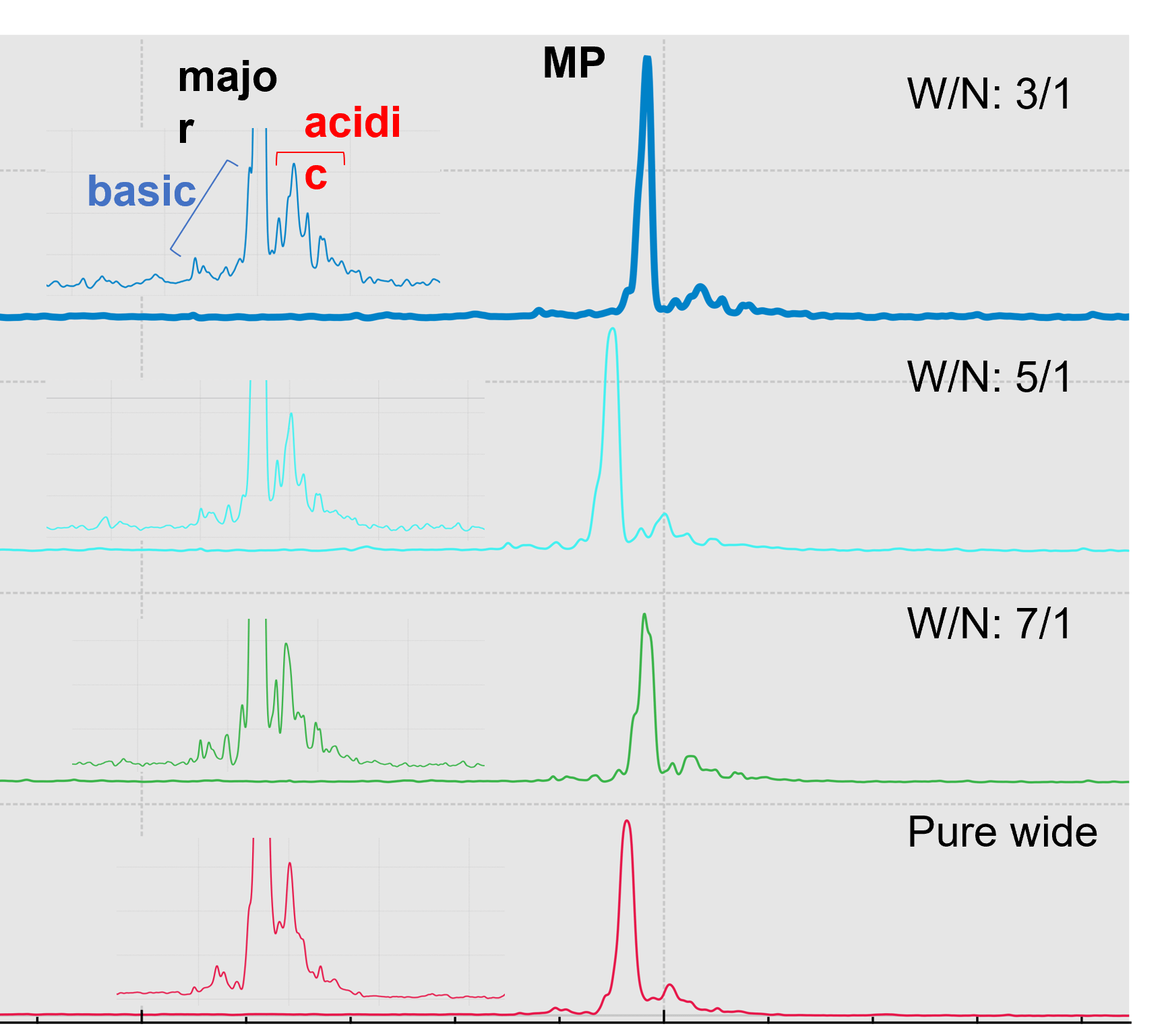 Click to enlarge
Click to enlarge