Determination of full, partial and empty capsid ratios for adeno-associated virus (AAV) analysis
Tingting Li, Tie Gao, Hongxu Chen, Zuzana Demianova, Fang Wang, Mukesh Malik, Jane Luo, Handy Yowanto, Sahana Mollah
SCIEX, Brea, CA, USA
Abstract
There are multiple technologies being used concurrently for determining the ratios of empty or partially filled capsids along with the full AAV such as analytical ultracentrifugation (AUC), transmission electron microscopy (TEM), etc. However, these traditional methodologies have their own set of challenges and hence drive a need for a parallel technique which is faster and easier to perform. This technical note demonstrates a robust capillary isoelectric focusing (cIEF)-based method for AAV full and empty capsids analysis. The results show excellent resolution between full and empty capsid as well as potential partial capsid peaks for determination of their ratios. The results from this methodology correlate well with orthogonal approaches such as anion exchange high performance liquid chromatography (AEX-HPLC), although AEX-HPLC provides less resolving power for these species and can struggle to quantify a smaller abundant peak vs. a closely eluting dominant peak.
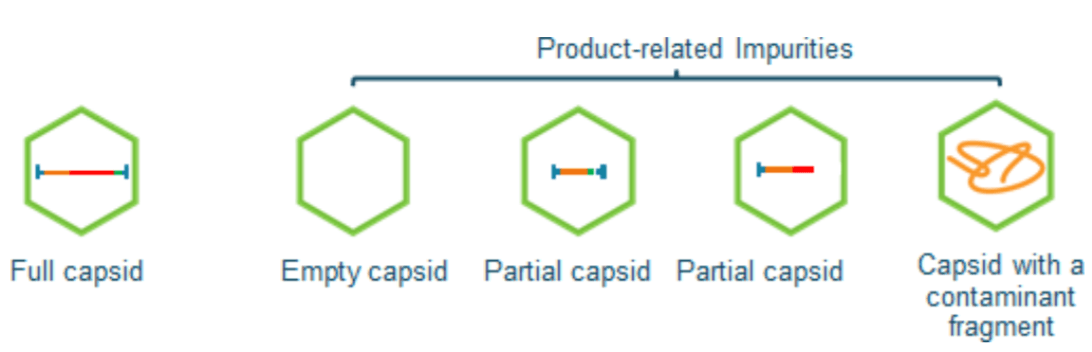
Introduction
Adeno-associated virus (AAV) is one of the most commonly used delivery vehicles (vectors) in gene therapy development. AAV is a combination of proteins (capsids) forming a closed shell encompassing a single-stranded DNA called a transgene.1 Manufacturing of AAV vectors produces three types of capsids empty, partial, and full. Considered manufacturing impurities are empty and partial AAVs because they either lack the genomic material or contain only fragments of the genome. The desired product is the full AAV because it includes the full length of the desired genomic material. The presence of these impurities could affect the efficacy and safety of AAV vector products because of their risk of increased immunogenicity of the end product. Additionally, it can inhibit the transduction of full AAVs by competing for vector binding sites on the transfected cells.2 Therefore, it is essential to determine the amount of these impurities (partial and empty AAVs), as well as the desired drug product (full AAV). Moreover, the ratio of full and empty is a critical quality attribute requirement for any AAV production process and quality control. There are multiple technologies currently being used to assess the full and empty ratio of AAV, such as analytical ultracentrifugation (AUC), transmission electron microscopy (TEM), to name a few. 3,4,5 However, these traditional methodologies have their own set of challenges hence driving the need for an orthogonal technique that is faster and easier to perform.
This technical note demonstrates a capillary isoelectric focusing (cIEF)-based method to separate AAV full and empty capsids and assess their ratio. The results shown demonstrate excellent resolution between full and empty AAV as well as potential partial capsid species allowing the assessment of their ratios. It is also capable of analysis of different serotypes of AAV. The results from this methodology correlate well with orthogonal approaches such as Anion Exchange-High Performance Liquid Chromatography (AEXHPLC). However, AEX-HPLC provides less resolving power for these species and can struggle to quantify a smaller abundant peak vs. a closely eluting dominant peak.
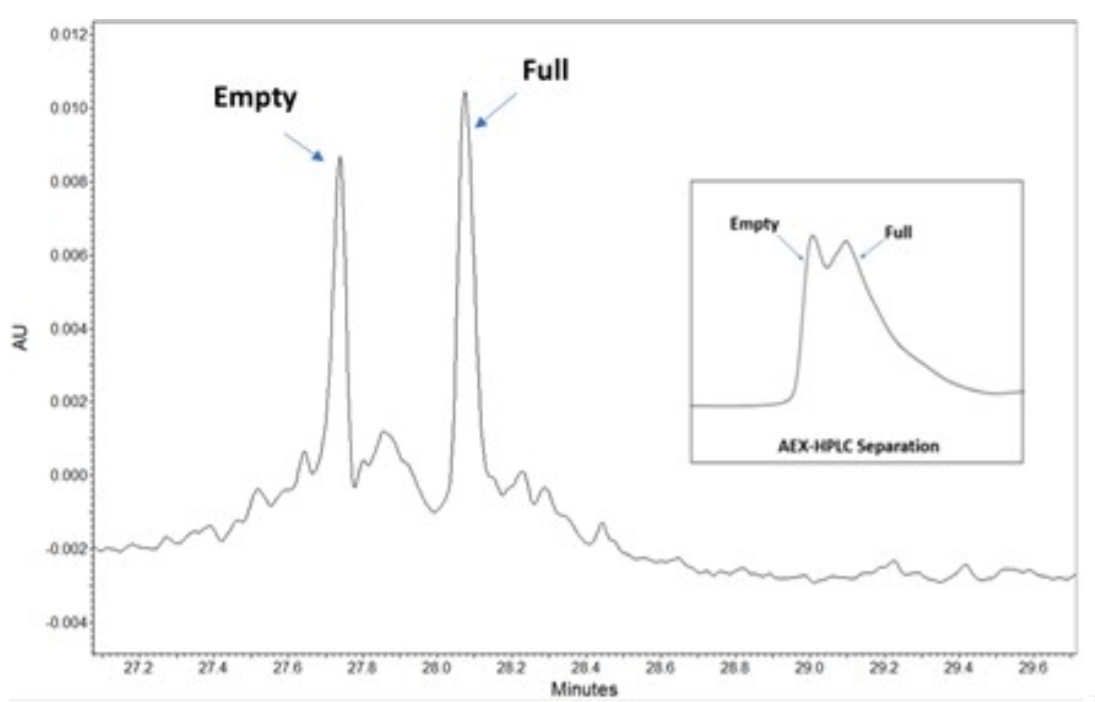
Figure 2. Separation of AAV serotype 5. Shown is an electropherogram with well resolved peaks between the empty and full capsids with 65nL of 1x1013 GC/ml sample load. The inset shows chromatogram of a 30ul load of the same sample analyzed with anion exchange chromatography.
Key Features
- A high resolution cIEF platform approach, with method optimization workflows for improved separation of AAVs of different serotypes with minimal pI differences (<0.1 pH unit)
- cIEF profiles and pI values can be determined and used for AAV vector identification
- Provides rapid analysis time with less than 1 hour per sample compared to traditional methods such as AUC and EM which can take days
- This method offers a good correlation of full and empty AAV ratio with orthogonal technologies such as AEX-HPLC.
Methods
cIEF Experimental
Instrumentation: All cIEF experiments were performed using a PA 800 Plus Pharmaceutical Analysis System (SCIEX, Brea, CA) equipped with a UV detector and a 280 nm filter (P/N 969136), as shown in Figure 1. Data were collected and analyzed using 32 Karat™ Software. The capillary was N-CHO capillary (SCIEX, P/N 477601) with 20 cm effective length and 30.2 cm total length. The separations were performed at 20° C with the anode at the sample introduction end of the capillary.
Sample Preparation and cIEF Separation Solutions: The cIEF gel (P/N 477497) and cIEF peptide marker kit (PN A58481) were purchased from SCIEX. Broad and narrow range ampholytes were purchased from GE Healthcare. The solutions used in the preparation of the cIEF sample master mix, as well as the separation electrolytes, were prepared according to the cIEF application guide6.
AAV Serotypes: Proprietary Serotype AAV Samples. A set of two AAV samples of proprietary serotype were used. Sample #1 is the sample with enriched empty capsids, while sample #2 is the sample with enriched full capsids. These two samples were concentrated by 20 times using Amicon Ultra 0.5mL Centrifugal Filters from EMD Millipore. Ten microliters of each AAV sample was mixed with 240 μL of Master Mix solution and transferred to sample vials for cIEF analysis.
AAV Serotype 5 Sample. An AAV5-CMV-GFP with a titer ~1X 1013 GC/mL (Cat# SL100819, Lot# AAV62019) from SignaGen Laboratories was used. The sample was prepared by mixing 3 μL of AAV5 with 24 μL of master mix and transferred to a nanoVial (SCIEX, P/N 5043467) for cIEF analysis.
AAV Serotype 8 (Full and Empty) Sample. Full and Empty AAV 8 samples were purchased from Vigene Biosciences (Lot# 2019.09.12). The titer of empty enriched packaged AAV8 of pAVCMV-GFP sample was 5.10 X1012 GC/mL, and the titer of full enriched packaged AAV8 of pAV-CMV-GFP sample was 1.10 X1013 GC/mL. Three microliters of each AAV8 sample was mixed with 24 μL of master mix and transferred to a nanoVial (SCIEX, P/N 5043467) for cIEF analysis.
AAV Serotype 9 Sample. An AAV9-CMV-GFP(Cat# SL100840) sample from SignaGen Laboratories was also used with a titer at 3.12 x1013 GC/mL. 3 μL of AAV 9 sample was mixed with 24 μL of master mix and transferred to a nanoVial (SCIEX, P/N 5043467) for analysis on a PA 800 Plus Pharmaceutical Analysis System.
The AAV5-CMV-GFP (Cat# SL100819, Lot# AAV62019) sample from SignaGen Laboratories was also analyzed using a CIMac SO3-0.1 AAV Analytical Column from BIA separations (PN 110.6157-1.3) on an ACQUITY UPLC H-class PLUS System from Waters Corporation for the AEX-HPLC analysis following the instruction of AAV Analytical Column7 for AAV full and empty capsid ratio comparison of orthogonal technologies.
Results and Discussion
Existing Methods to Separate AAV Full and Empty Capsids
There are multiple methods to determine the ratio between the full and empty AAV. One such approach is determining the percentage of the full capsids in the total capsids by dividing the number of genome vectors derived from the existing qPCR data by the total capsid number obtained from the ELISA data.8 However, this method lacks sufficient data accuracy and precision. Another way is to determine the protein and DNA content in the samples by spectrophotometric means using the optical density of AAV samples at 260 nm and 280 nm.9 This approach is simple, rapid, and easy to operate. However, it requires high purity of the AAV sample to minimize the interference of the impurities with UV absorbance at 260 nm and 280 nm. A third alternative and perhaps the most complex is AUC.3. Indeed, AUC can separate full, partial, and empty AAVs. However, analysis is length, expensive, and it demands a large sample amount, data interpretation requires expertise, making this technique unattractive in a GMP environment. TEM is another often used technology in the industry, and it could reliably count the full and empty particles as a population.4,5 However, it is challenging to distinguish the partial capsids, and it is timeconsuming for data interpretation to meet the need for timely results required in quality control.
Perhaps the most popular analytical technique used for the quantitative determination of AAV full/empty is Ion exchange chromatography10. However, this method requires a large number of samples and is serotype- dependent. Furthermore, it could not distinguish between partial, full, and empty AAV. Additionally, it lacks the necessary resolving power between full and empty AAV imparing the quantitation of these species, resulting in inaccurate full and empty ratio. Mass spectrometry based approaches such as charge detection mass spectrometry (CDMS) has been recently shown to be able to separate full, partial and empty capsids.11 However, this system is not commercially available.
Figure 3. cIEF Results of a low AAV titer of a Proprietary Serotype.
cIEF-Based Separation of AAV Full and Empty Capsids
The developed cIEF approach aims to efficiently separate the analytes by their pI (isoelectric point) values. The principle ofthe method is simple. The full capsids have pI values lower than their empty counterparts due to negative charges from the ssDNA they encapsulate. Therefore, our cIEF approach separates the AAVs and their undesired product-related impurities by taking advantage of their different isoelectric points. Figure 3 shows the cIEF results of AAV samples of a proprietary serotype. Two samples of the same AAV product with different amounts of full and empty capsids were analyzed. Sample #1 was enriched with empty capsids, while sample #2 was enriched with full capsids. The cIEF profiles of the proprietary AAV samples mobilized between pI marker 7.0 and 10.0. The empty capsid peak is mobilized at a higher pI value while the full capsid peak at lower pI value. Meanwhile, some potential partial capsid peaks are mobilized between the peaks corresponding to the empty and full capsid. Notably, the cIEF profiles were consistent with the profiles obtained by analytical ultracentrifugation (data not shown).
The calculated pI difference between the full and empty AAV serotype 8 is about 0.1 pH units imposing a separation challenge. The broad range ampholytes failed to provide sufficient baseline resolution compromising the quantitation of the full and empty capsids. However, baseline resolution was achieved by using a mixture of broad and narrow range ampholytes. The dark blue circles in Figure 4, highlighted the empty and full capsid peaks of the AAV8 samples.
The single peak in front of the circle was identified as an impurity peak from one of the pI markers, since it was also observed in a blank injection with pI markers. Notably, higher intensity peaks corresponding to the empty capsid was observed in the empty capsid-enriched sample. In comparison, higher intensity of full capsid peaks were found in the full capsid-enriched sample as expected. Finally, we demonstrate in figure 4, that by expertly utilizing the narrow range ampholyte, it is possible to achieve excellent baseline resolution of the AAV full and empty capsids. This high-resolution cIEF method also may also give insights into the charge heterogeneity characteristics of AAVs. Indeed, multiple peaks were observed for the empty as well as full capsids of this set of AAV 8 samples, which could indicate the charge heterogeneity. Further experiments are needed to validate this claim.
Figure 4. cIEF Results of AAV Serotype 8 Samples. A isAAV8 Empty(E) and B is AAV8 Full (F).
Table 1. Calculated pIs of Separated Peaks Using cIEF Method in Figure 3.
Similarly, a mixture of wide and narrow range pH ampholytes were used to optimize the cIEF separation of AAV serotype 9 sample. Instead of unresolved peaks observed at pI 7.3 and 7.5 from using the wide range pH ampholyte (Figure 5a), multiple resolved peaks between pI 7.3 to 7.6 were observed when using the mixture of pH ampholytes. Thus providing more valuable information on the abundance of partial/variants present with the full capsid.
Figure 5. Shown are the electropherogram a cIEF analysis of the AAV9 sample with a) analysis using a wide pH ampholyte and b) a mix of wide and narrow ampholyte.
Repeatability Analysis
To determine the analytical reproducibility of the method the AAV9 serotype sample was run 5 times to obtain the %RSD for the peak area and migration time. As shown in figure 6, we get excellent reproducibility with %RSD of <5% and <2% for the peak area and pI value, respectively.
Distinct pI values for AAV identification
It is worth noting that the pI values of the AAV capsid peaks can be quantitatively determined based on the calibration curve of internal pI markers. The pI value of the AAV8 samples was approximately 7.1(pI value data not shown in Figure 4), while the pI of the AAV samples with proprietary serotype was about 9.0 (Table 1). These results demonstrate that this assay can potentially be used as an AAV vector identification method based on the profile and pI values.
Figure 6. 5 replicate analysis of Serotype AAV 9. The %RSD for peak area and pI are <5% and <2%,respectively.
Determination of AAV Full /Empty Capsids Ratio
The ratio of full/partial/empty capsids can be calculated based on the corrected peak areas of the separated capsid peaks in the cIEF electropherograms. The relative content of the full and empty capsids of AAV 8 samples separated in Figure 4, is summarized in Table 2.
Table 2. F/E AAV Determination of AAV8 Samples Separated in Figure 4.
Figure 2 shows the full and empty separation profile of AAV5 sample by both cIEF (left panel) and AEX- HPLC (right panel). As observed, the cIEF method nicely resolves the full from the empty, as well as the partial capsids, compared to the AEX-HPLC method, which showed poor resolution. Additionally partially filed AAVs were clearly observed in the cIEF methods, whereas in the HPLC method it was not. Hence to compare the orthogonality of these two analytical separation approaches, we included the Area % of both partial and full in cIEF and counted as total full AAV and compared with AEX-HPLC. Their absorbance at 280 nm for both technologies was also evaluated for comparison. The results in Table 3, reveals that the ratio of the full and empty capsids determined by cIEF correlates well with that of the AEXHPLC method.
Table 3. Comparison of AAV5 Full and Empty Capsids Determination Using cIEF and AEX-HPLC Methods.
* It is the sum of full and partial peaks for cIEF
It is worth noting that absorbance at 280 nm of AAV capsids, is prone to over-estimation due to the contribution of extra UV absorbance of the genetic materials in full capsids. Hence a correction factor using the molar extinction coefficients of the full and empty capsids at different wavelengths is needed. For comparative analysis with other orthogonal techniques such as TEM and AUC, using this correction factor for cIEF analysis will improve the accuracy for the quantitative determination of full capsids for the various serotypes.
Conclusion
Empty/ versus full is an important CQA that needs to be monitored throughout the development and production of the viral vector based therapy. While a number of techniques can be used for empty full analysis, there are still limitation to these workflows. This technical note demonstrates a robust cIEFbased method for the separation and analysis of AAV full and empty capsids of different serotypes. The pI profiles can be determined and used for AAV identification. The utilization of the optimal mixture of wide and narrow pH range ampholytes can efficiently improve the separation of AAV samples with small pI differences between full, partial and empty capsids. The sample analysis time for this method is rapid, less than 1 hour per sample. The analysis is performed on a well-validated and automated cIEF-based platform to obtain reliable and reproducible results across multiple serotypes. Making this method amenable for usage in release testing.
References
- https://en.wikipedia.org/wiki/Adeno-associated_virus
- Wright, J. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines 2014,2, 80-97
- Burnham B, Nass S, Kong E, Mattingly M, Woodcock D, Song A, Wadsworth S, Cheng SH, Scaria A, O’Riordan CR (2015) Analytical ultracentrifugation as an approach to characterize recombinant adeno-associated viral vectors. Hum Gene Ther Methods 26(6):228–242.
- Chen, H. (2007). Comparative Observation of the Recombinant Adeno-Associated Virus 2 Using Transmission Electron Microscopy and Atomic Force Microscopy. Microscopy and Microanalysis, 13(5), 384-389
- Z. Hong Zhou. Seeing Engineered Loops in a Gene Delivery Vehicle by cryoEM. Structure, Volume 20, Issue 8, 8 August 2012, Pages 1286-1288
- Goricar,B, Peljhan, S., Gagnon, P. Estimation of empty and full AAV10 particle ratio using the multi-angle light scattering detector;poster(2019) (https://www.biaseparations.com/en/library/posters/1012/esti mation-of-empty-and-full-aav10-particle-ratio-using-the- multiangle-light-scattering-detector)
- Grimm D. Kern A. Pawlita M., et al. Titration of AAV-2 particles via a novel capsid ELISA: Packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6:1322– 1330.
- Sommer JM, Smith PH, Parthasarathy S, Isaacs J, Vijay S, Kieran J, Powell SK, McClelland A, Wright JF (2003) Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol Ther 7(1):122– 128.
- Martin Lock, Mauricio R. Alvira, and James M. Wilson. Analysis of Particle Content of Recombinant Adeno-Associated Virus Serotype 8 Vectors by Ion-Exchange Chromatography. Human Gene Therapy Methods.Feb 2012.56-64.
- Pierson, Elizabeth E. and Keifer, David. Z. and Asokan, Aravind and Jarrold, Martin F. Resolving Adeno-Associated Viral Particle Diversity With Charge Detection Mass Spectrometry. Anal. Chem. 2016 88 (13), 6718-6725.
Request a full version by clicking the link below:
https://info.sciex.com/aav-technote-request
 Click to enlarge
Click to enlarge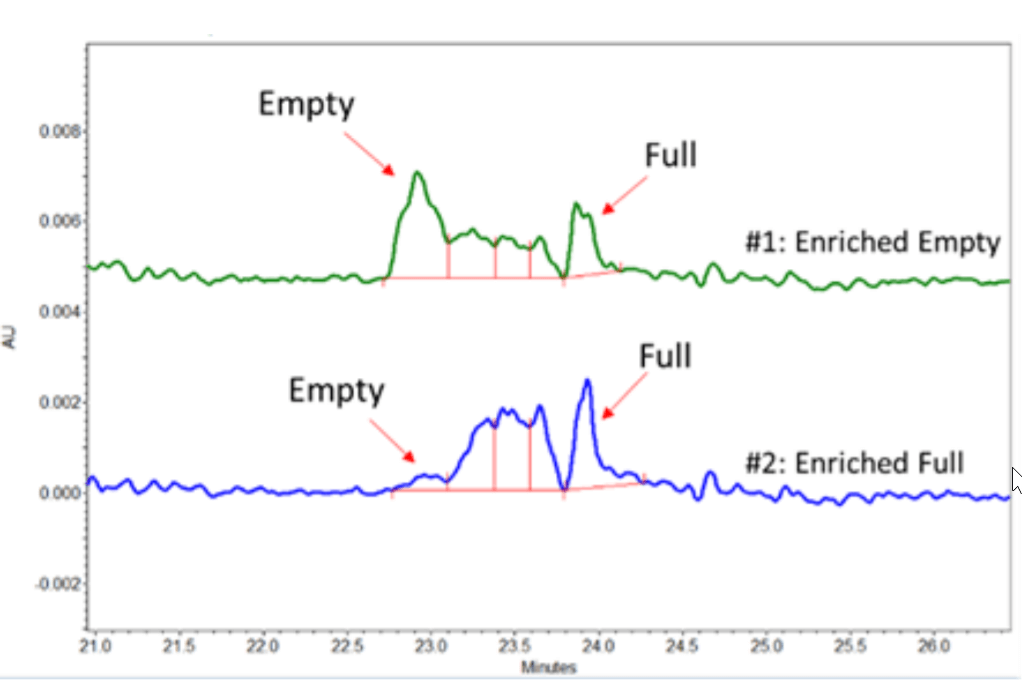 Click to enlarge
Click to enlarge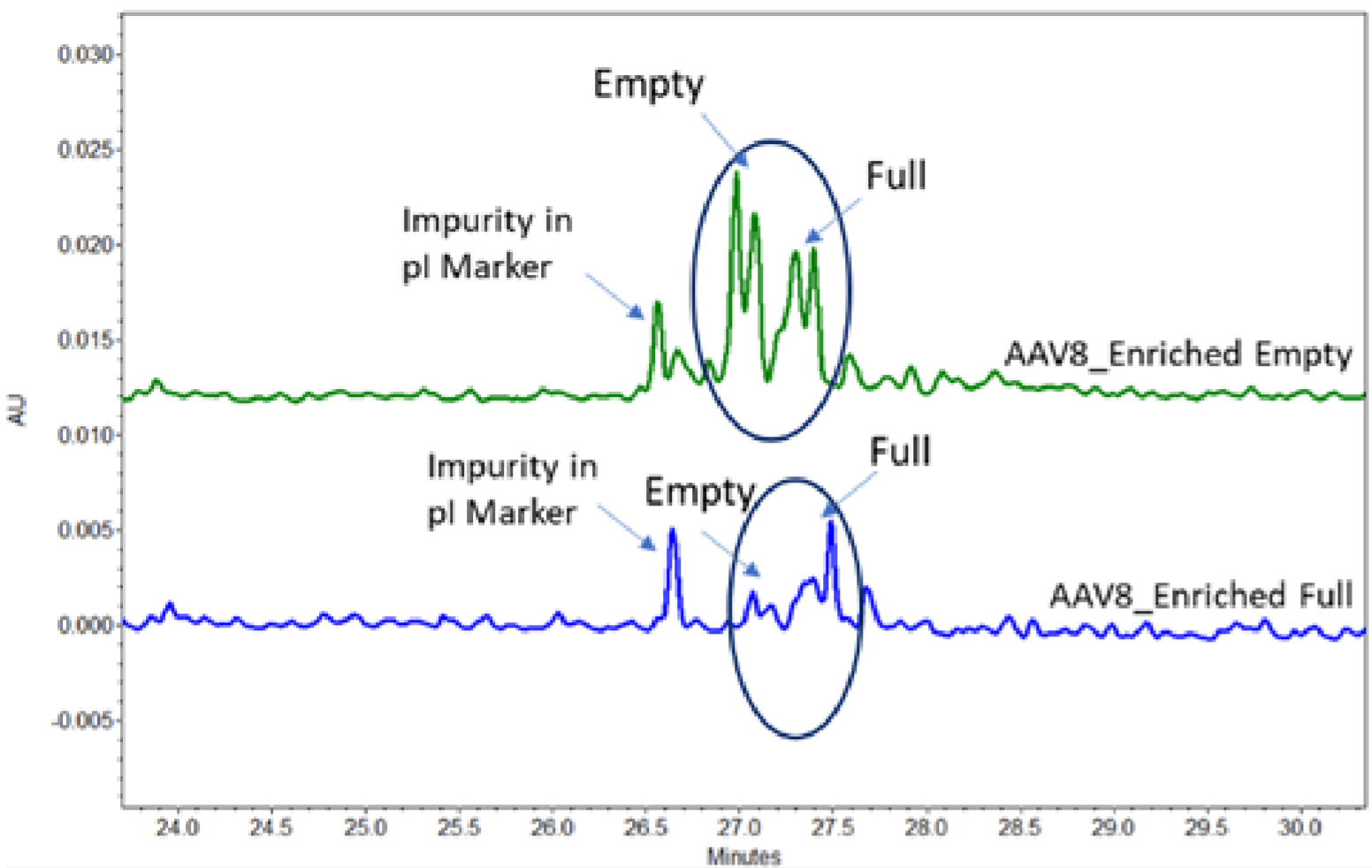 Click to enlarge
Click to enlarge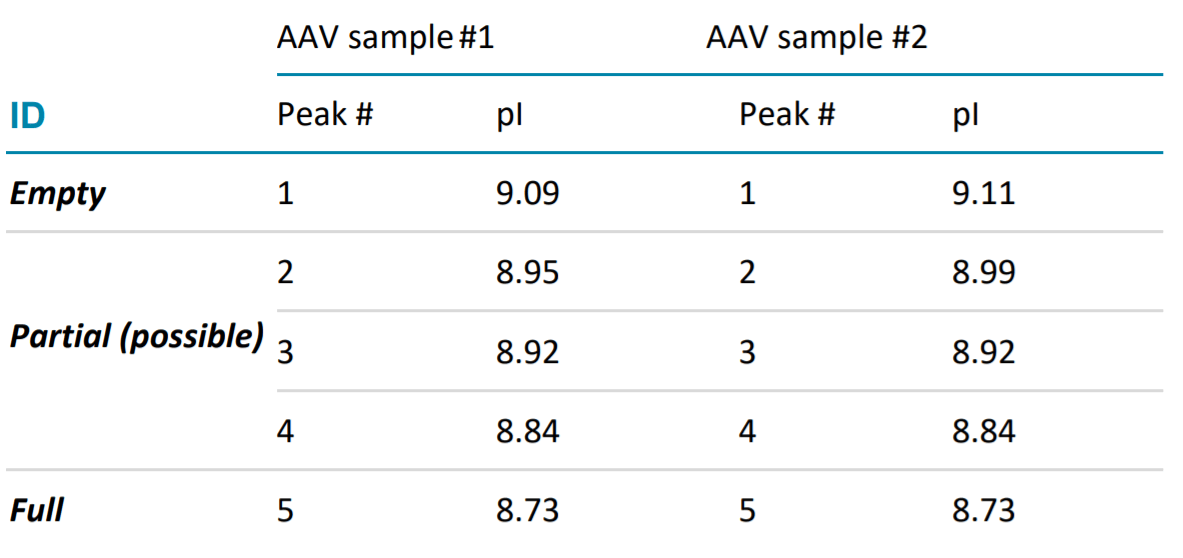 Click to enlarge
Click to enlarge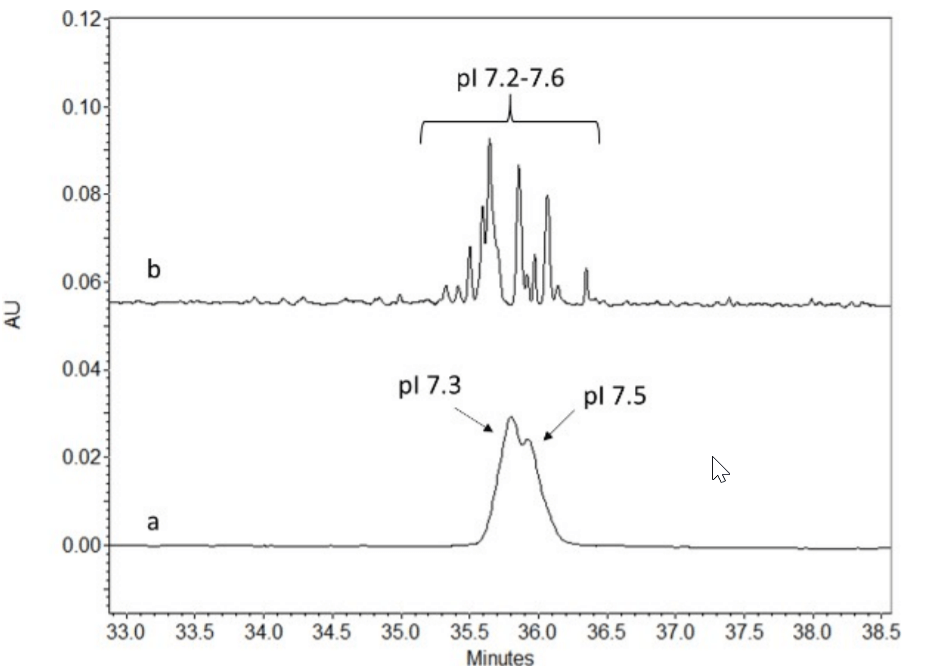 Click to enlarge
Click to enlarge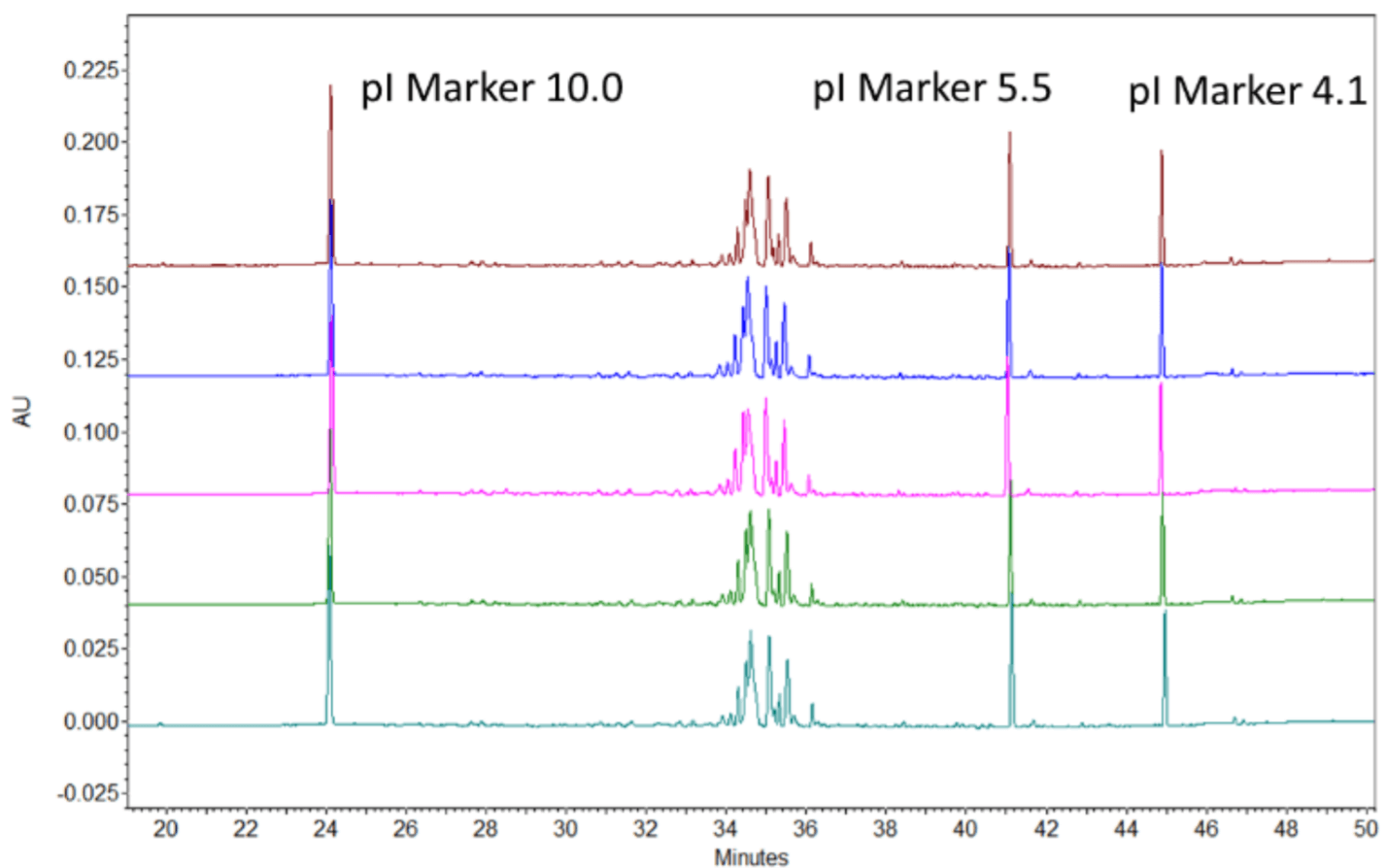 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge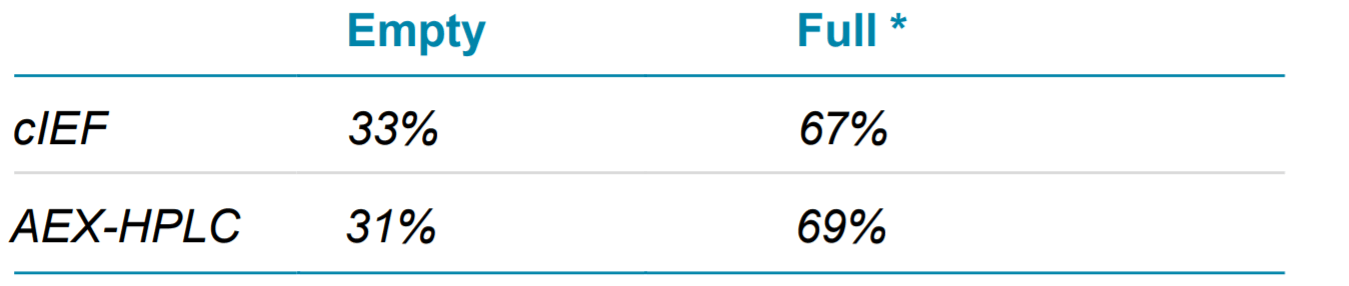 Click to enlarge
Click to enlarge