Measuring per- and polyfluoroalkyl substances in baby formula powder: A quantitative LC-MS/MS approach
Karl A. Oetjen and Simon Roberts
SCIEX, USA
Abstract
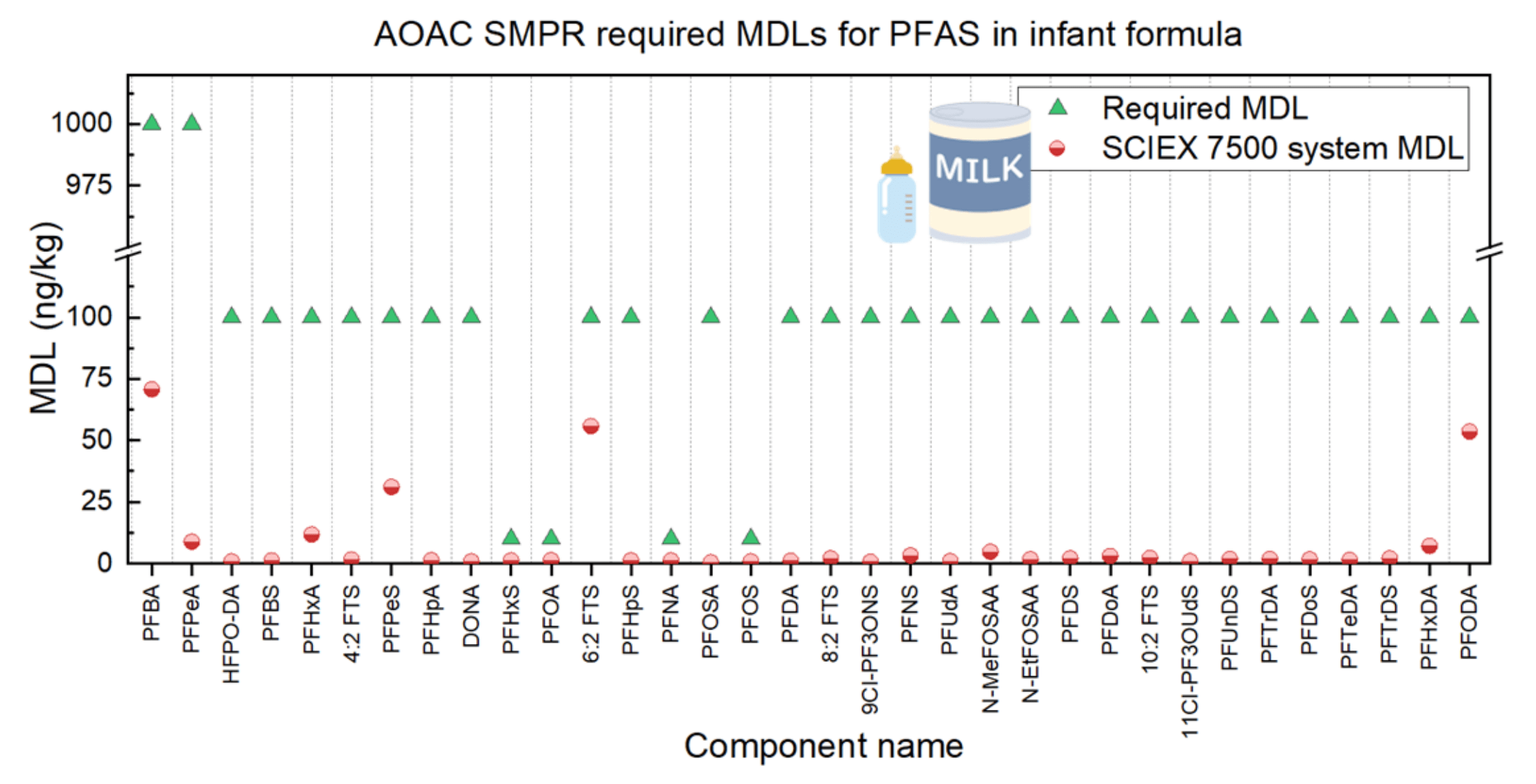
This technical note describes the trace-level analysis of per- and polyfluoroalkyl substances (PFAS) in infant formula following the AOAC Standard Method Performance Requirements (SMPRs) for PFAS in produce, beverages, dairy products, eggs, seafood, meat products and feed. Using the SCIEX 7500 system, the method detection limits (MDLs) for 34 different PFAS in baby formula powders ranged from 0.5 ng/kg for PFOSA to 71 ng/kg for PFBA. These results met the required MDLs outlined in the AOAC SMPRs (Figure 1).
Introduction
PFAS are a group of man-made chemicals used in various consumer products, including food packaging.1 Some PFAS compounds have been linked to adverse health effects, including developmental issues, immunotoxicity and certain types of cancer. Measuring PFAS in baby formula is crucial to protect infants from potential health risks associated with these chemicals.1 To achieve this, sensitive MDLs are required. In this study, we conducted a thorough survey of various baby formulas, seeking out the "cleanest" formula for use in our spiking experiments. The MDLs were then calculated following the guidelines outlined in 40 CFR 136 Appendix B and by performing a total of 7 spikes and 7 blank tests. Finally, we compared the PFAS concentrations detected in the baby formula samples with the concentrations reported in the literature and with PFAS levels found in human breast milk.
Figure 1. Method detection limits (MDLs) observed using the SCIEX 7500 system compared to the MDLs required by the AOAC SMPRs for PFAS in produce, beverages, dairy products, eggs, seafood, meat products and feed.
Key benefits of the method for PFAS analysis of infant formula using the SCIEX 7500 system
- All calculated MDLs were below the AOAC target limits of quantitation (LOQs) with MDLs for 27 PFAS <5 pg/g
- Contamination was a significant concern due to the concentration factor during the sample extraction process. To address this issue, SPE cleaned water was used for extraction.
- All but 1 baby formula had detectable levels of PFOS (MDL 0.84 pg/g, mean 3.4 pg/g, max 5.5 pg/g
Methods
Sample preparation. The infant formula samples were prepared by placing 5 g of baby formula powder and 100 µL of isotopically labeled surrogate in a polypropylene tube that was pre-cleaned with acetonitrile (Figure 2). Then, 15 mL of LC-MS-grade water that was pre-cleaned using a Strata-XAW solid-phase extraction (SPE) column (Phenomenex, P/N: 8B-S038-FCH) was added to the tube, along with 10 mL of LC-MS-grade acetonitrile that was previously screened for PFAS. QuEChERS was then performed using 6 g of magnesium sulfate (MgSO4) and 1.5 g of sodium acetate. The samples were shaken vigorously before being centrifuged for 5 minutes at 10,000 rcf. A 1.75 mL aliquot of the supernatant was transferred to a pre-cleaned 2 mL centrifuge tube and dispersive SPE (dSPE) was performed using 150 mg of MgSO4, 50 mg of primary and secondary amine (PSA) and 50 mg of graphitized carbon black (GCB). The sample was then shaken for 30 seconds before being centrifuged at 10,000 rcf for 5 minutes. A 1 mL aliquot of supernatant was transferred to an autosampler vial and evaporated under a gentle stream of nitrogen. The sample was reconstituted with 200 µL of 80:20, methanol/water and isotopically labeled standards were added before injection.
Figure 2. Simplified sample extraction procedure for PFAS in baby formula powder.
Liquid chromatography. Liquid chromatography was performed using a Shimadzu LC-40 system equipped with a delay column (Luna Omega PS C18, 5 μm, 50 × 3 mm, Phenomenex, P/N: 00B-4753-Y0) at a flow rate of 0.8 mL/min. The injected sample volume was 20 µL and separation was conducted using a Luna Omega PS C18 analytical column (3 µm, 150 × 3 mm, Phenomenex, P/N: 00F-4758-Y0).
The AOAC SMPRs require the method to demonstrate that cholic acid interferences, including taurodeoxycholic acid (TDCA), taurochenodeoxycholic acid (TCDCA) and tauroursodeoxycholic acid (TUDCA), do not co-elute with the PFOS m/z 499→80 MS/MS transition. Baseline separation of these compounds must be achieved between the cholic acids and all PFOS isomers or these interferences must be removed before chromatographic separation during the extraction or cleanup procedures. Additionally, the MS/MS transition m/z 499→124, present in all 3 cholic acids, was monitored to confirm the separation or removal of cholic acids from samples. The LC composition and gradient were optimized to achieve this (Figure 3). Mobile phase A was water with 10mM ammonium acetate and mobile phase B consisted of 80% acetonitrile, 15% methanol and 5% water with 5mM ammonium acetate. Mobile phase B was initially held at 5% for 0.5 minutes and then ramped to 35% within 1 minute. Then, mobile phase B was ramped to 50% over 3 minutes, increased to 80% at 7 minutes and eventually raised to 95% by 10 minutes, where it was held for 2 minutes. The gradient was then returned to 5% and was held for 3 minutes.
Figure 3. Chromatographic separation of PFOS from the cholic acid interferences, TDCA, TCDCA and TUDCA.
Mass spectrometry. The samples were analyzed on a SCIEX 7500 system operated in negative ionization mode. The gas pressures used included CUR 40 psi, GS1 35 psi, GS2 70 psi and CAD 12 psi. The source temperature was set to 325°C and the ion spray voltage was -1300 V. A total of 34 PFAS compounds were monitored using optimized MRM parameters, with a minimum of 10 scans required per peak. For all analytes, 2 transitions were monitored, except for PFBA and PFPeA, which required an orthogonal method for identification (high-resolution mass spectrometry, secondary column chemistry) because they lack a secondary transition.
Controlling contamination
The challenge of PFAS analysis arises from the widespread presence of PFAS compounds in commonly used laboratory items. These substances can inadvertently contaminate samples during testing, significantly impacting the accuracy of analyses, especially when striving for the low concentrations required by the AOAC SMPRs. To achieve the desired MDL of 5 ppt, the water used in sample preparation must contain <0.22 pg/mL of each PFAS due to the concentration step involved in the described sample extraction process.
To ensure limited contamination, extraction blanks were performed for each reagent and material in the extraction procedure, including the solvents, QuEChERS and dSPE kits. Several batches of QuEChERS and dSPE kits were contaminated with PFAS, particularly PFOA and 6:2 FTS. Additionally, 6:2 FTS was identified as a major source of contamination in the water used for QuEChERS extraction (Figure 4). To mitigate the levels of 6:2 FTS, SPE was used to clean the water before extraction and dSPE materials were screened prior to use.
Figure 4. Example of 6:2 FTS contamination in the water used for the QuEChERS extraction.
Method detection limit study
The MDLs were established following the guidelines set by the National Environmental Laboratory Accreditation Conference (NELAC) and 40 CFR Part 136. The method described here involved processing at least 7 spiked samples and 7 method blank samples. The spiking concentration depended on the required MDL, which ranged from 5 pg/g to 100 pg/g. The samples designated for determining the MDL were prepared in 3 batches on separate calendar dates and were subsequently analyzed on 3 separate calendar dates. These samples were then analyzed alongside 7 laboratory blanks, all of which were analyzed on a minimum of 3 different days.
The MDLs observed were <5 pg/g (ng/kg) for 27 PFAS compounds and ranged from 0.5 pg/g for PFOSA to 71 pg/g for PFBA (Figure 1). PFBA, 6:2 FTS, PFODA and PFPeS exhibited occasional blank contamination, resulting in MDLs higher than the other 30 compounds. However, these elevated MDLs were below the MDLs required by the AOAC SMPRs.
The compounds with the lowest required MDLs, set at 10 pg/g, included PFOS, PFOA, PFNA and PFHxS. In this study, the MDLs for these specific compounds were determined to be 0.83 pg/g, 1.34 pg/g, 1.25 pg/g and 1.22 pg/g, respectively.
Method recovery study
The AOAC SMPRs require the recovery of PFOS, PFOA, PFHxS and PFNA to be within 65%–135%. To calculate the recovery, 7 matrix spike samples were spiked with isotopically labeled PFAS analogs and native PFAS. For isotope dilution quantitation, the response of the target compound was standardized by comparing it to the response of either its isotopically labeled counterpart or the isotopically labeled analog of another compound with similar chemical properties and retention time. The calculated recoveries for these compounds were 94% ± 8%, 97% ± 5%, 73% ± 5% and 96% ± 6 %, respectively. The recoveries for the remaining compounds ranged from 70% to 123% (Figure 5).
Figure 5. Compiled recovery results from matrix spike samples.
Analysis of baby formula powders and breast milk
To facilitate comparison with breast milk, concentrations in pg/g of formula powder were converted to concentrations in pg/mL of prepared baby formula, following formula-specific instructions (Figure 6). All tested baby formulas contained at least 1 PFAS compound and all but 1 showed detectable levels of PFOS (mean 3.4 pg/g, max 5.5 pg/g). Hypoallergenic baby formula, which includes hydrolyzed milk, had the fewest PFAS compounds detected, yet it exhibited the second-highest levels of PFBA (92.5 pg/g). Notably, the PFAS concentrations in the tested baby formulas were lower than the average concentrations reported in the literature for both breast milk and baby formula.1-4 However, the breast milk sample analyzed demonstrated PFAS concentrations similar to the mean values reported in the literature3,4 but were significantly higher than those found in the baby formula samples in this study.
Figure 6. Concentration of PFAS in baby formula powders and breast milk.
Conclusion
In summary, this technical note demonstrates how the sensitivity of the SCIEX 7500 system enables a method capable of achieving the performance requirements outlined in the AOAC SMPRs for the analysis of PFAS in infant formula. In this study, the MDLs for 34 PFAS compounds were determined, with most compounds detected at <5 pg/g. Additionally, a method recovery study was performed, which achieved accurate results within the specified range for key PFAS compounds. Finally, PFAS concentrations in baby formulas were analyzed and were detected at lower levels than reported in the literature, whereas PFAS were present in breast milk at concentrations consistent with the literature.
References
- Fujii, Y., Yan, J., Harada, K.H., Hitomi, T., Yang, H., Wang, P. and Koizumi, A., 2012. Levels and profiles of long-chain perfluorinated carboxylic acids in human breast milk and infant formulas in East Asia. Chemosphere, 86(3), pp.315-321. https://doi.org/10.1016/j.chemosphere.2011.10.035
- Macheka, L.R., Olowoyo, J.O., Mugivhisa, L.L. and Abafe, O.A., 2021. Determination and assessment of human dietary intake of per and polyfluoroalkyl substances in retail dairy milk and infant formula from South Africa. Science of The Total Environment, 755, p.142697. https://doi.org/10.1016/j.scitotenv.2020.142697
- Tao, L., Ma, J., Kunisue, T., Libelo, E.L., Tanabe, S. and Kannan, K., 2008. Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States. Environmental science & technology, 42(22), pp.8597-8602. https://doi.org/10.1021/es801875v
- Zheng, G., Schreder, E., Dempsey, J.C., Uding, N., Chu, V., Andres, G., Sathyanarayana, S. and Salamova, A., 2021. Per-and polyfluoroalkyl substances (PFAS) in breast milk: concerning trends for current-use PFAS. Environmental science & technology, 55(11), pp.7510-7520.
https://doi.org/10.1021/acs.est.0c06978
 Click to enlarge
Click to enlarge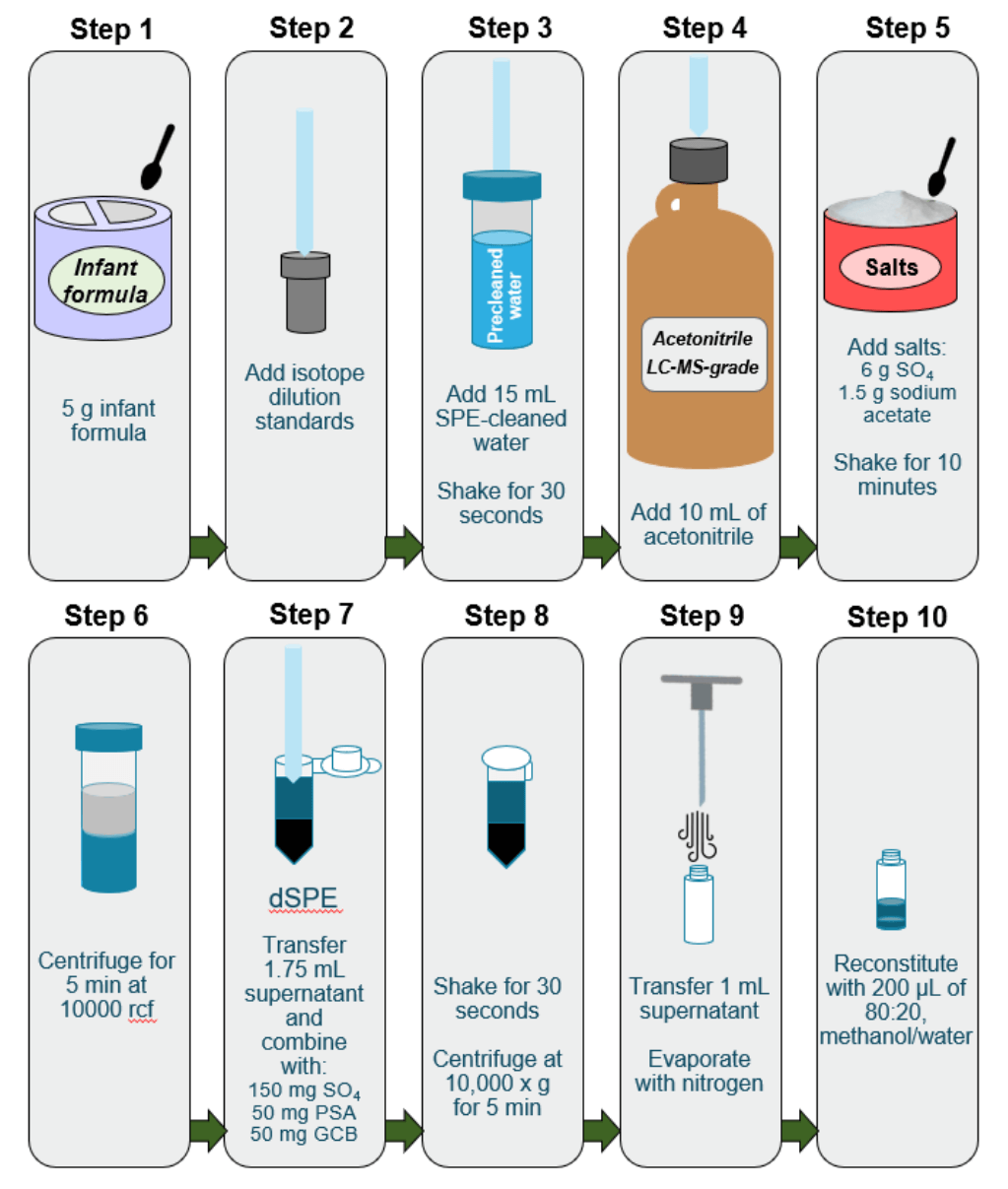 Click to enlarge
Click to enlarge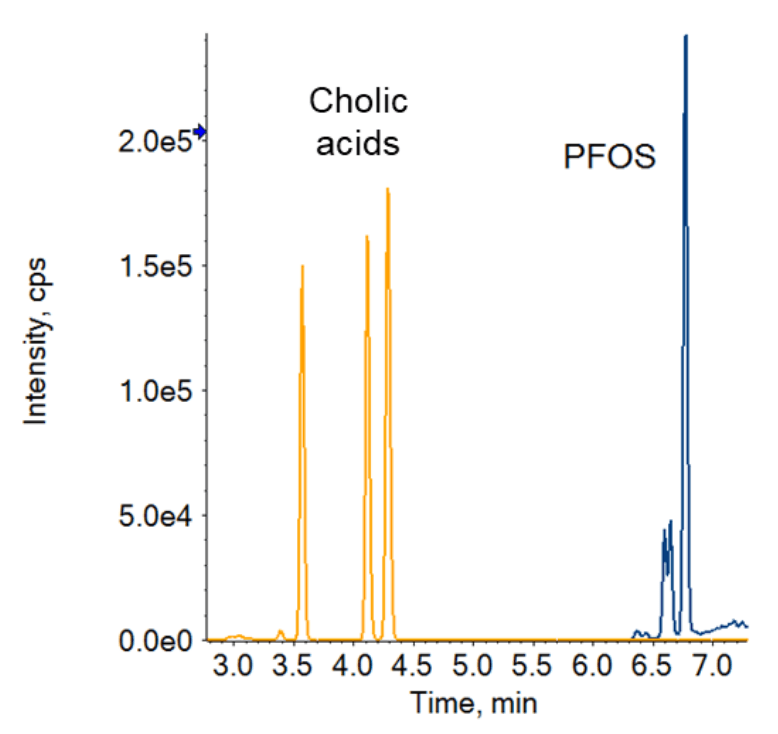 Click to enlarge
Click to enlarge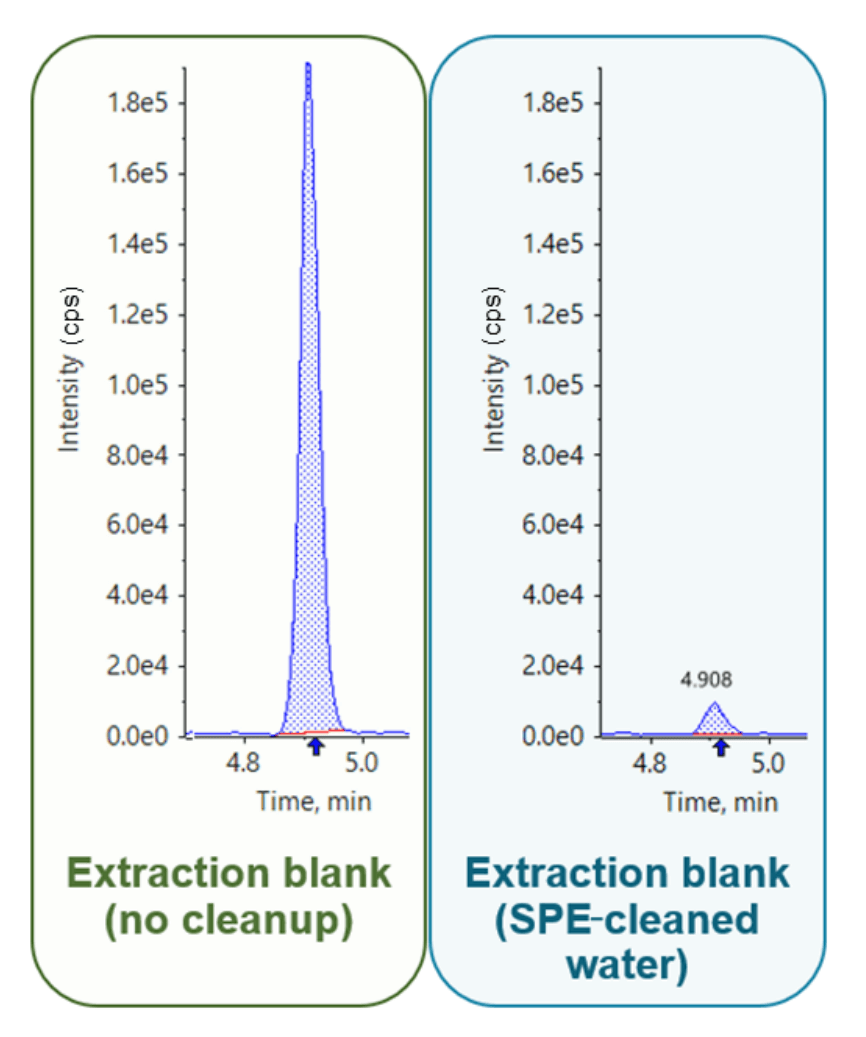 Click to enlarge
Click to enlarge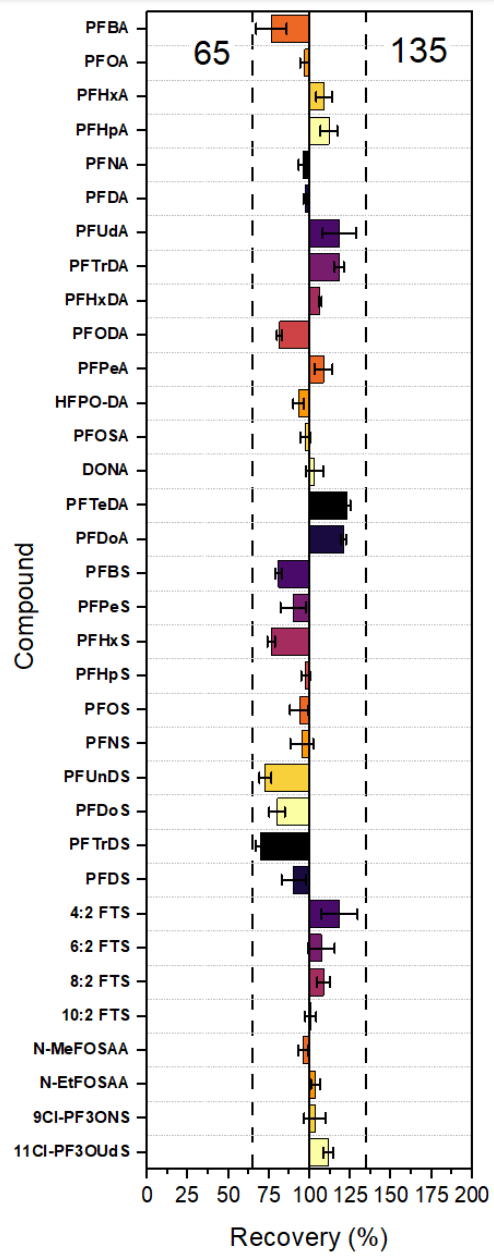 Click to enlarge
Click to enlarge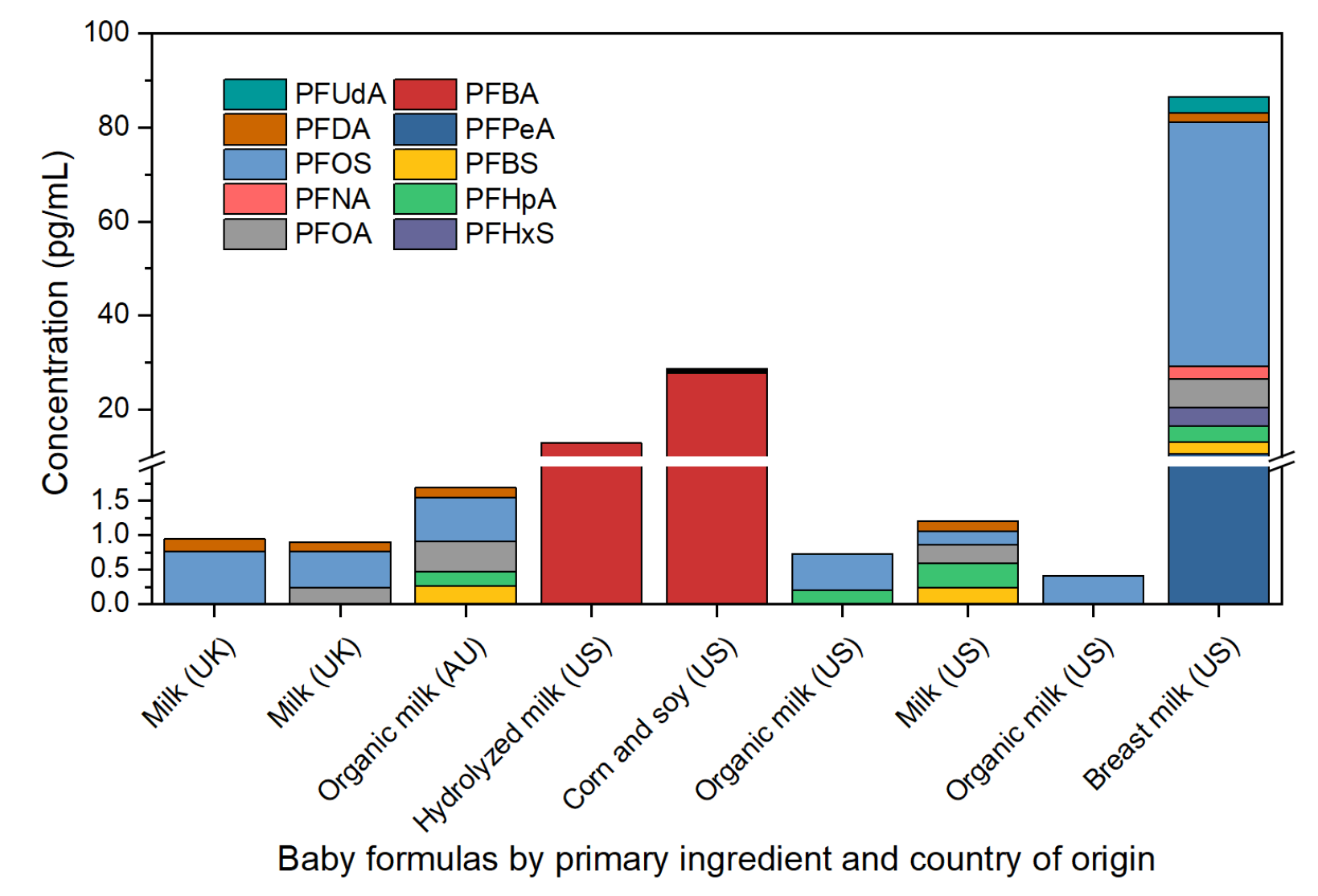 Click to enlarge
Click to enlarge