Quantitation of per- and polyfluoroalkyl substances (PFAS) in foodstuffs
Michael Scherer1 , Jessica Smith2 , Jack Steed2 , Daniel McMillan2 , Jianru Stahl-Zeng1
1SCIEX, Germany; 2SCIEX, UK
Abstract
This technical note describes a simple and sensitive method using the SCIEX 7500 system for the quantitation of per- and polyfluoroalkyl substances (PFAS) in different foodstuffs. The method is in response to the new maximum residue levels (MRLs) outlined in the Commission Regulation (EU) 2022/2388 of 7 December 2022.1 The high sensitivity of the SCIEX 7500 system2 enabled the development of an LC-MS/MS method that requires only a 2 µL food extract injection volume, while still achieving good quantitative performance in the sub µg/kg range. Application of this method to the analysis of pork liver, honey and egg extracts provided by Fera Science Ltd. resulted in excellent agreement with the previously measured reference levels.
Introduction
PFAS compounds can enter the food chain either through environmental contamination or migration from food packaging. Based on a risk assessment of PFAS contamination in food on human health, the European Food Safety Authority (EFSA) established a group tolerable weekly intake (TWI) of 4.4 ng/kg body weight for the sum of PFOS, PFOA, PFNA and PFHxS. As a result, MRLs and total limits of the four PFAS were set, to regulate their presence in certain foodstuffs of animal origin as a protective measure against dietary exposure.1 Due to the lack of data for many foods, the Commission also recommended continued PFAS monitoring in a broad range of foodstuffs to assess the need for further regulatory actions.3 PFAS measurements in foods require low limits of quantitation (LOQs),4 which necessitate robust analytical performance and increased instrument sensitivity. Here, the sensitivity of the SCIEX 7500 system was leveraged to allow small sample injection volumes to reduce matrix interferences and to allow the use of solvent-based calibration to simplify quantitation for a wide variety of different food matrices.
Key features of PFAS analysis in foodstuffs using the SCIEX 7500 system
- The sensitivity of the SCIEX 7500 system allowed for a small injection volume of 2 µL to reduce matrix interferences in food extracts, while still achieving sub µg/kg LOQs based on signal-to-noise (S/N).
- Acceptable precision (%CV <15%), linearity (r 2 >0.995) and ion ratio tolerance (±30%) were achieved using the external solvent-based standard calibration curve.
- Calculated concentrations for PFAS compounds in an EURL proficiency test sample (pork liver) were found to be within the satisfactory range (±2 z-score)
- Positive detection of PFAS was achieved in the unspiked blank food extracts, although the levels were all below their corresponding MRLs
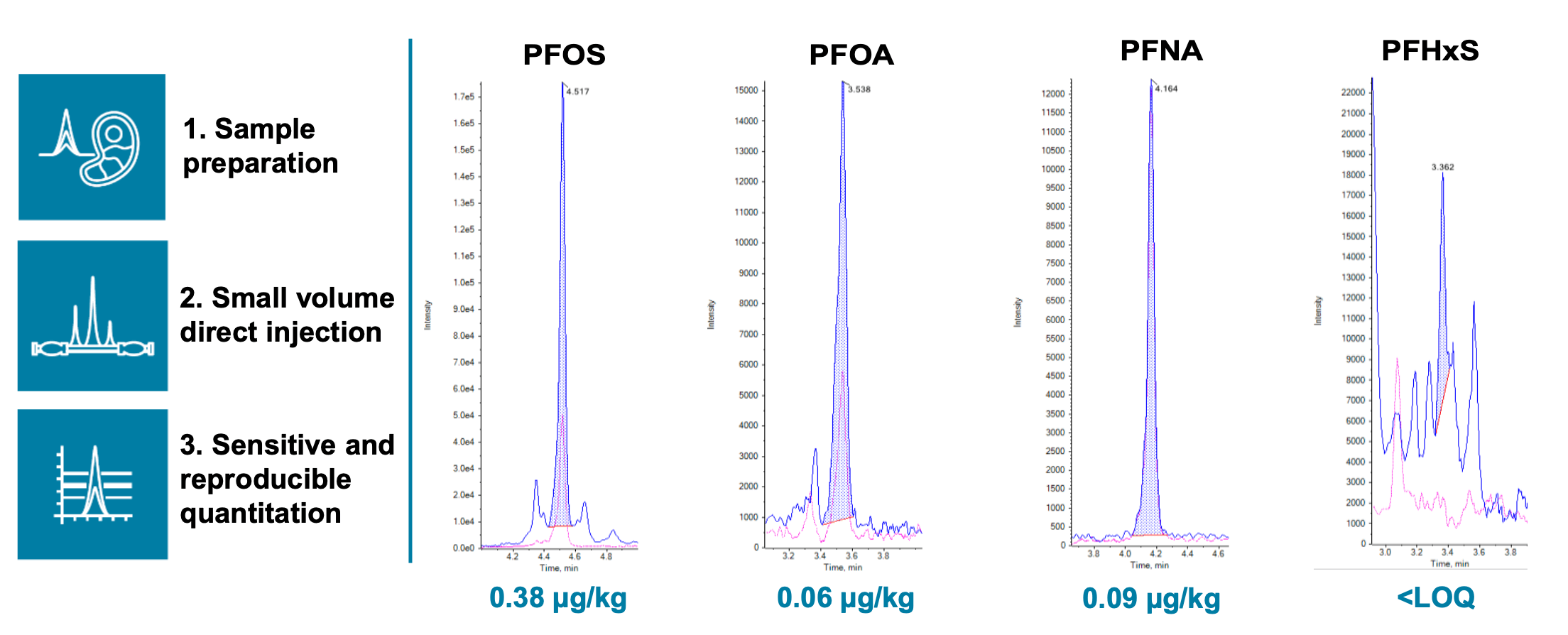
Figure 1. Extracted ion chromatograms (XICs) of the 4 regulated PFAS compounds (PFOS, PFOA, PFNA and PFHxS) with their concentrations measured in an unspiked pork liver extract. Using the SCIEX 7500 system, LOQs of 0.01 ng/mL were achieved for PFOS and PFNA, and 0.02 ng/mL for PFHxS and PFOA, in diluent. This was based on an ion ratio tolerance of ±30% for the quantifier and qualifier transitions and S/N >10. Because of the small injection volume of 2 µL, the reduction of matrix effects in food enabled the use of solvent-based calibration to derive these low LOQs, which in turn allowed for positive detections of PFAS in even the unspiked food extracts.
Methods
Samples: Sample preparation and spiking experiments were conducted by Fera Science Ltd. according to their in-house standard operating procedure (SOP). The SOP involved samples being extracted with basified methanol and diluted prior to clean-up on a WAX SPE cartridge. All consumables and solvents were tested for PFAS contamination prior to use.
Chromatography: Chromatographic separation was performed using a Phenomenex Luna Omega PS C18, 100 Å, 100 mm x 3 mm, 3 µm analytical column (PN: 00D-4758-Y0) and a Phenomenex Gemini C18, 110 Å, 50 mm x 3 mm, 5 µm delay column (PN: 00B-4435-Y0) (Figure 2). An injection volume of 2 µL and a flow rate of 0.6 mL/min were used. Mobile phase A was 2mM ammonium acetate in water and mobile phase B was 2:1 acetonitrile/methanol. Gradient conditions are shown in Table 1.
Table 1. Chromatographic gradient program
Mass spectrometry: The analysis was performed using the SCIEX 7500 system operated with electrospray ionization in negative ion mode.
Data acquisition and processing: Acquisition and processing were performed using SCIEX OS software version 3.0.
Chromatography and ultra-trace sensitivity of PFAS compounds in solvent standards
Good chromatographic separation was achieved for all target PFAS (Figure 2), including the branched and linear isomers of PFHxS and PFOS (Figure 3). Although the MRLs apply to the sum of linear and branched stereoisomers, chromatographic separation can help distinguish their individual contribution to any observed detection by using isomer-specific standards.
With PFAS quantitation being continuously challenged by the diversity of food matrices, it is paramount to develop robust and sensitive analytical methods that can achieve low LOQs and overcome matrix interferences. Because of the sensitivity of the SCIEX 7500 system, only 2 µL sample injection volumes were required to achieve the solvent-based LOQs shown in Figure 4 for the 4 regulated PFAS compounds (PFOS, PFOA, PFNA, PFHxS). The minimal matrix effects afforded by the small injection load on the instrument allowed for the use of solvent-based calibration, which is ideal for quantifying PFAS in a wide variety of food matrices. Table 2 summarizes the sub-ng/mL, solvent-based LOQ values that were achieved for all PFAS compounds in diluent. LOQ determination was based on the lowest solvent-based concentration achieving S/N values >10 and ion ratio tolerance of ±30% between the quantifier and qualifier ions. While these LOQs demonstrate the high instrument sensitivity of the SCIEX 7500 system, further validation is necessary to determine LOQs in matrix spikes.
Figure 2. Chromatogram of the target PFAS compounds in a 2 ng/mL standard prepared in solvent. Good chromatographic separation was achieved with the first eluting PFBA and the four regulated PFAS (PFHxS, PFOS, PFOA and PFNA), labeled above their corresponding peaks.
Figure 3. XICs of PFOS and PFHxS in a 1 ng/mL standard prepared in solvent. The XICs show chromatographic separation of both the branched and linear isomers of PFOS and PFHxS.
Figure 4. XICs for PFOS, PFOA, PFNA and PFHxS at their respective LOQs in solvent (100% methanol). LOQs of 0.01 ng/mL were achieved for PFOS and PFNA, and 0.02 ng/mL for PFHxS and PFOA, in diluent. This was based on an ion ratio tolerance of ±30% for the quantifier and qualifier transitions and S/N >10.
Table 2. LOQ of each compound analyzed in diluent, with peak-to-peak S/N added to show that each peak is quantifiable with S/N above 10x and % accuracy at the LOQ of 89.4%-118%. %CV of peak area was measured across 6 replicate injections of a 0.1 ng/mL standard in diluent and ranged from 1.3 to 15%.
Precision and linearity
Good linear performance (r 2 >0.995) was achieved for the solvent-based calibration curves for all target PFAS (Table 2). Average accuracy and precision were assessed for 6 replicate injections of a 0.1 ng/mL solvent standard and found to be within acceptable validation requirements (accuracy ±20% and peak area %CV <15%).
Method performance in a pork liver proficiency sample
A proficiency test (PT) for the determination of persistent halogenated organic pollutants, including PFAS, was organized by the European Union Reference Laboratory (EURL).5 The objective was to assess analytical performance and interlaboratory comparability. Pork liver extracts were spiked by Fera Science Ltd. and provided to SCIEX for analysis. Figure 5 shows the XIC comparisons of solvent blanks, unspiked and spiked pork liver extracts for the 4 regulated PFAS. Table 3 compares the concentrations of various PFAS in the liver PT samples measured by the SCIEX 7500 system against the average concentrations previously determined by the EURL interlaboratory study.5 The excellent agreement between the concentrations quantified by the external solvent-based calibration curve and the interlaboratory results demonstrates that ion suppression was minimal due to the small injection volume used (2 µL).
Furthermore, the SCIEX 7500 system provided sufficient sensitivity to detect and quantify the presence of the 4 regulated PFAS in even the unspiked pork liver extract, as shown in Figures 1 and 5.
Figure 5. XICs of the 4 regulated PFAS compounds in solvent blanks, unspiked and spiked pork liver extracts. No contamination was observed for the 4 regulated PFAS in the solvent blanks. Despite the small injection volume (2 µL) used, the peak intensities in the spiked pork liver extracts were high and incurred PFAS were detected in the unspiked extracts, demonstrating the high sensitivity of the SCIEX 7500 system.
Table 3. Comparison of PFAS concentrations in a spiked pork liver extract measured by SCIEX and the EURL PT results.
EU MRLs for PFAS compounds in foodstuffs
In addition to the PT samples, the 4 regulated PFAS were also detected in unspiked pork liver, egg and honey extracts. Table 4 shows the EU MRLs for pork liver and egg compared against the concentrations of PFOS, PFOA, PFNA and PFHxS measured in the unspiked food extracts. The detected PFAS compounds in the unspiked food extracts were lower than the EU MRLs for all analyte/matrix combinations except for PFOS in pork liver. This highlights the excellent sensitivity of the SCIEX 7500 system, which provided sufficiently low LOQs required by EU regulations.3
Table 4. Comparison of the EU MRLs for PFOS, PFOA, PFNA and PFHxS in pork liver and egg with the concentrations measured in the blank food extracts. There are currently no MRLs established for PFAS in honey. Quantitation is confirmed by two MRM transitions and an ion ratio tolerance of ±20%.
Conclusion
- Due to the high sensitivity of the SCIEX 7500 system, only a small injection volume (2 µL) of a food matrix extract was required, which was beneficial in reducing matrix interference in complex matrices
- Lower matrix suppression enabled the use of a single solvent-based calibration, which greatly simplified quantitation of PFAS in a variety of different food matrices
- Excellent precision (peak area %CV of 1.26–14.83%, n = 6), linearity (r 2 >0.99) and ion ratio tolerance within 30% were achieved for all target PFAS in the solvent standards
- The calculated concentrations of PFAS compounds in the spiked pork liver extracts were in excellent agreement with the EURL interlaboratory PT results
- The high sensitivity of the SCIEX 7500 system revealed sub µg/kg detection of PFAS even in the blank food extracts, although the levels were mostly below their corresponding EU MRLs, except for PFOS in pork liver
- Although internal standards were not used in this work, their use is highly recommended to compensate for recovery losses and matrix effects that are often present in complex food matrices
References
- Commission Regulation (EU) 2022/2388 of 7th December amending Regulation (EC) No 1881/2006 as regards to maximum levels of perfluoroalkyl substances in certain food stuffs. Official Journal L 318/38.
- SCIEX 7500 system. SCIEX product overview.
- Commission Regulation (EU) 2022/1431 of 24 August 2022 on monitoring of perfluoroalkyl substances in food. Official Journal L221/105.
- Guidance Document on Analytical Parameters for the Determination of Per-and Polyfluoroalkyl Substances (PFAS) in Food and Feed. Version 1.2- 11th May 2022.
- EURL Proficiency Test on the Determination of PCDD/Fs, PCBs, PBDEs, HBCDDs, PFASs and CPs in Pork liver 2022. EURL-PT-POP_2202-PL FOOD.
 Click to enlarge
Click to enlarge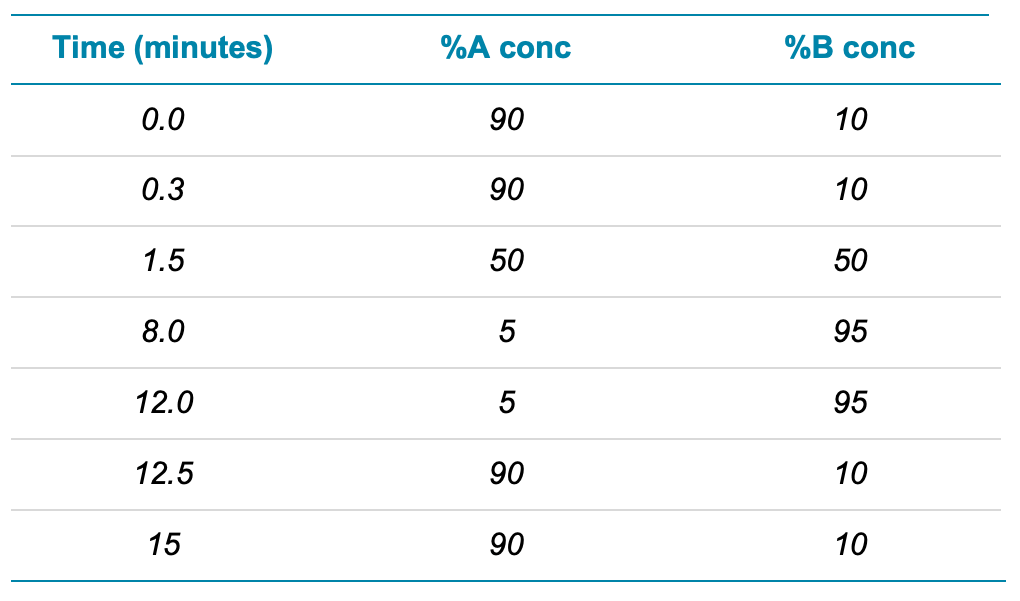 Click to enlarge
Click to enlarge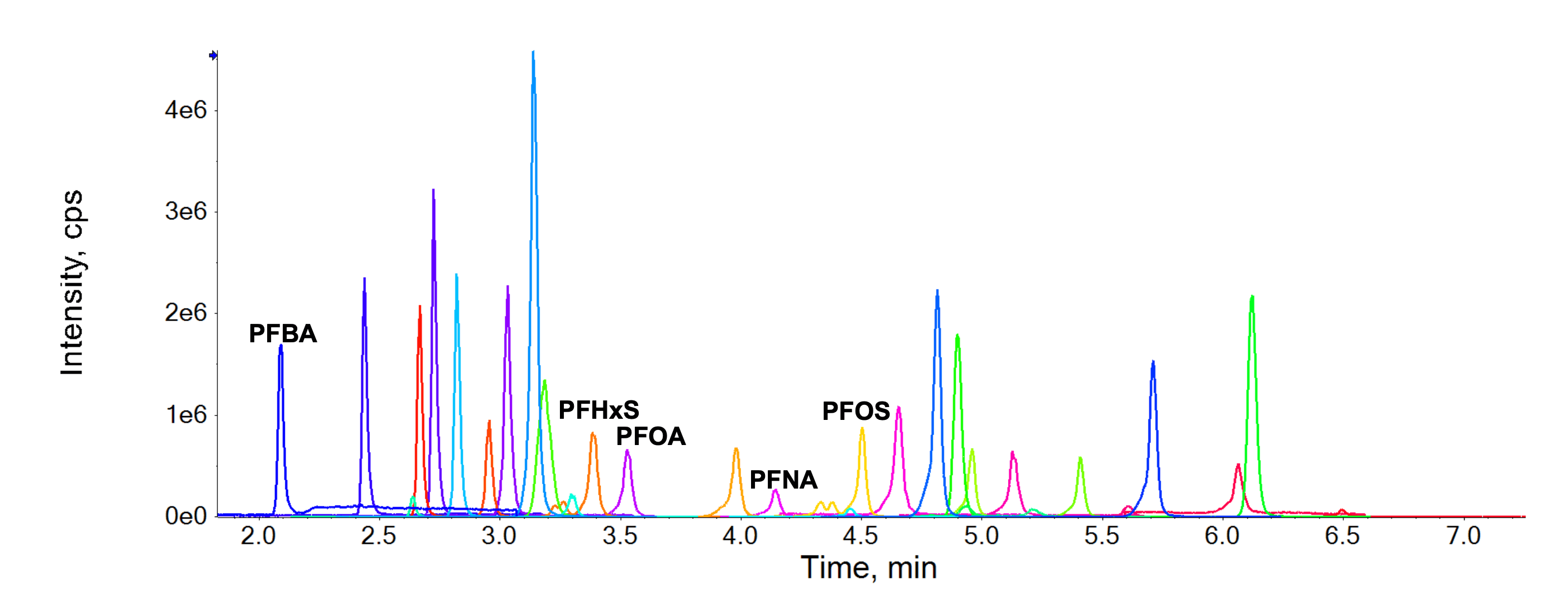 Click to enlarge
Click to enlarge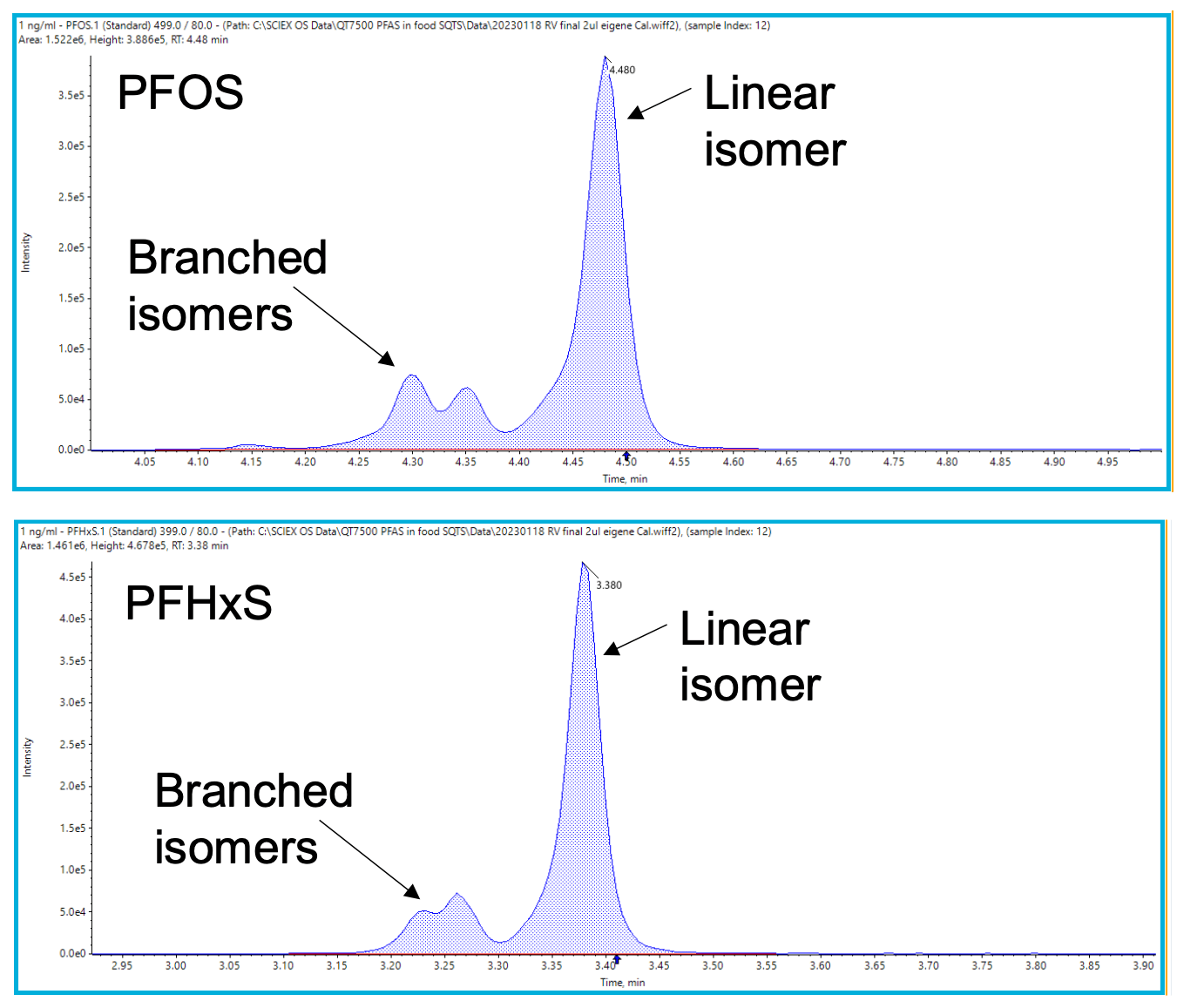 Click to enlarge
Click to enlarge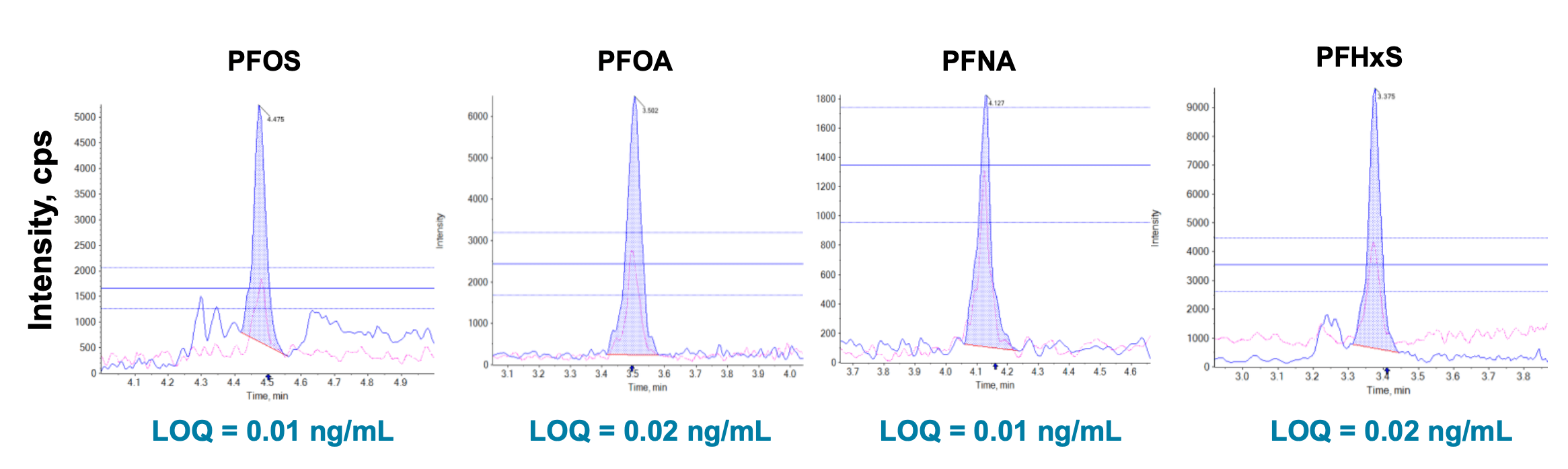 Click to enlarge
Click to enlarge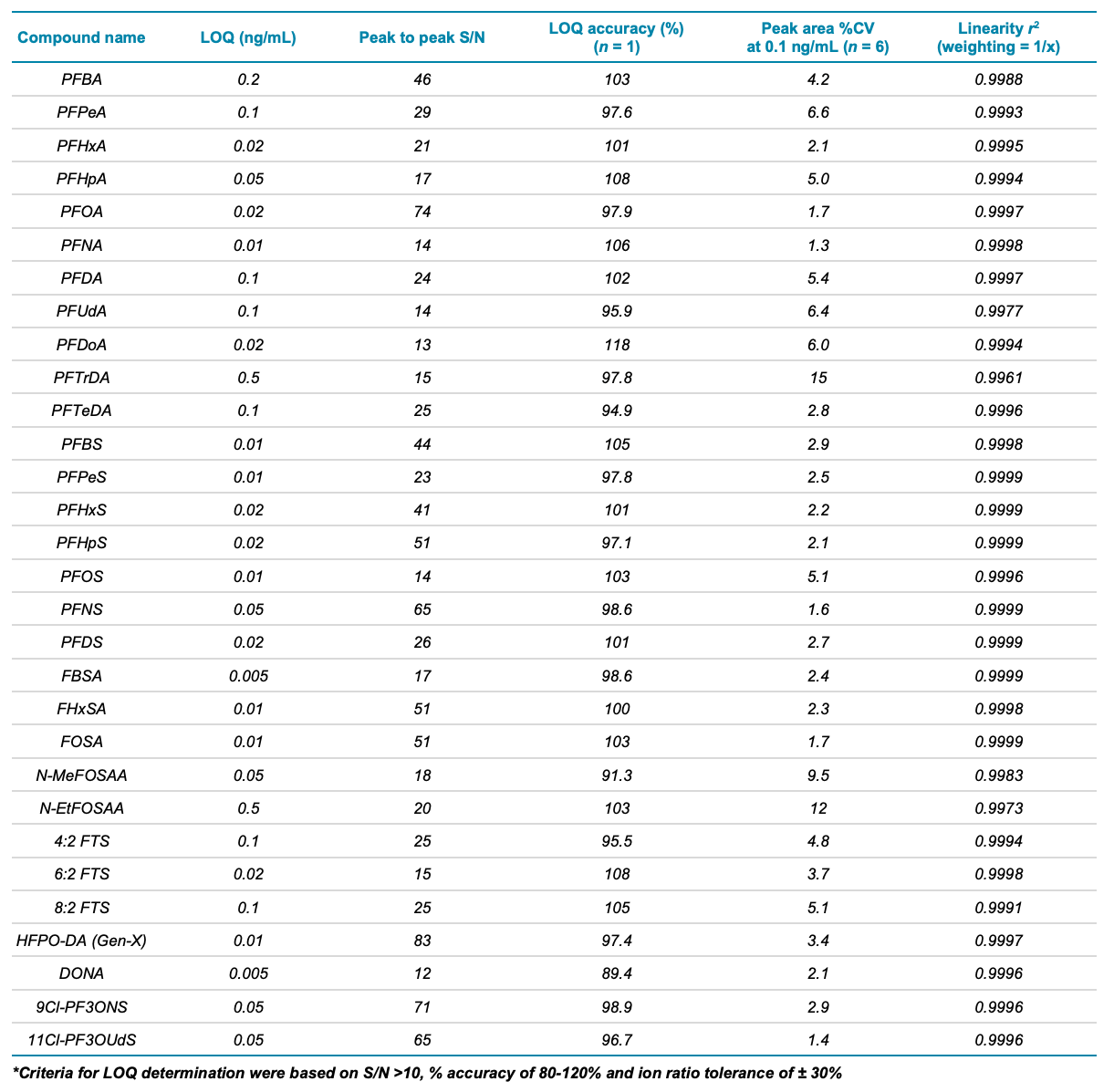 Click to enlarge
Click to enlarge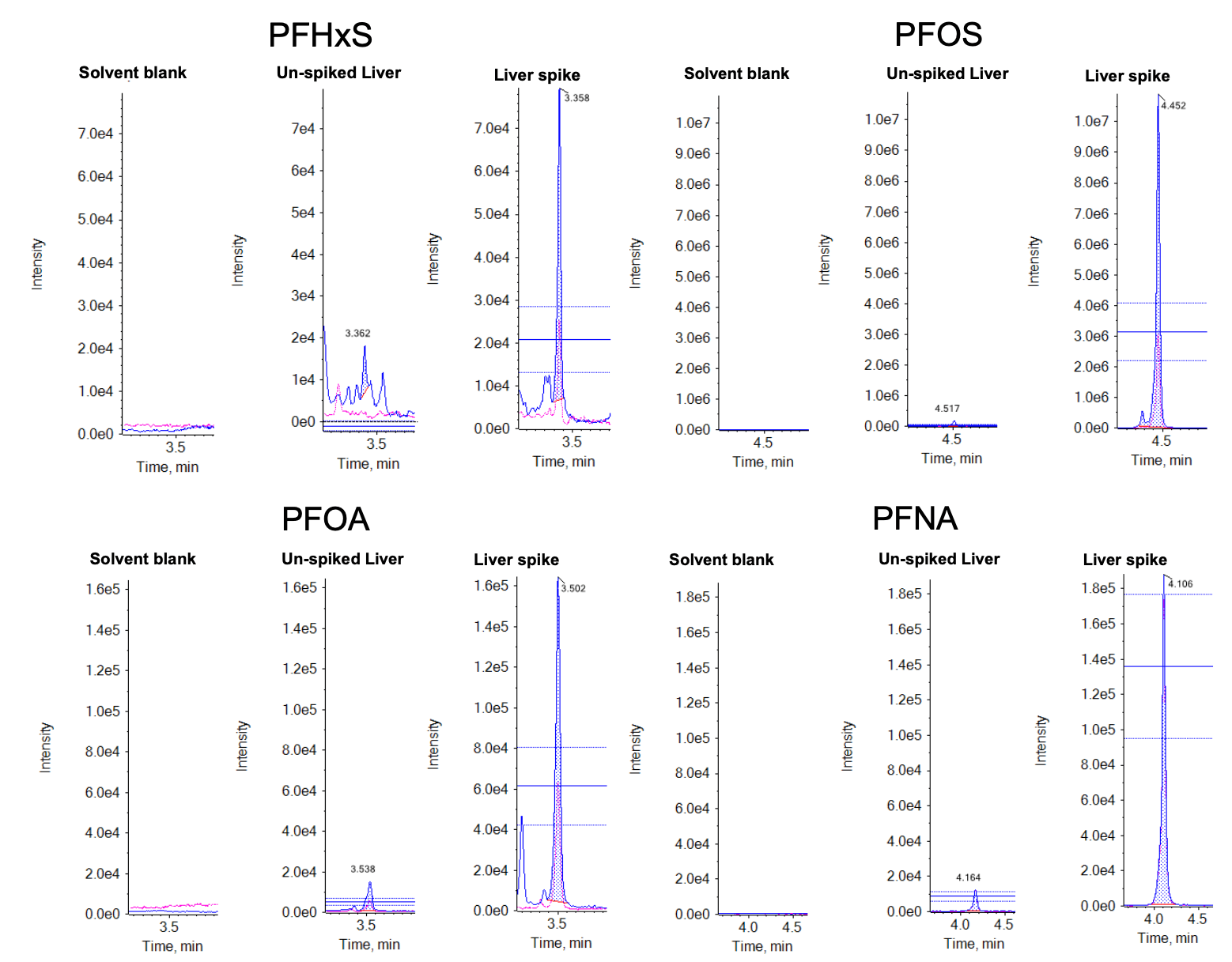 Click to enlarge
Click to enlarge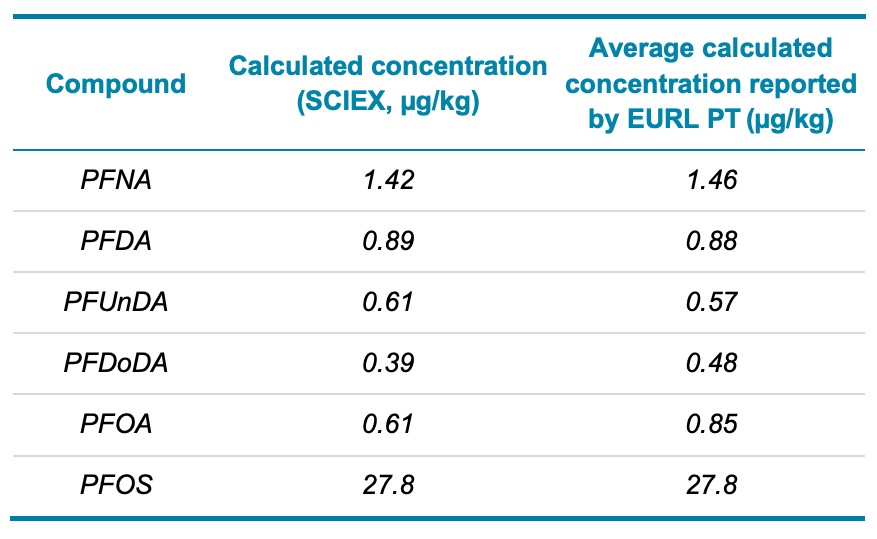 Click to enlarge
Click to enlarge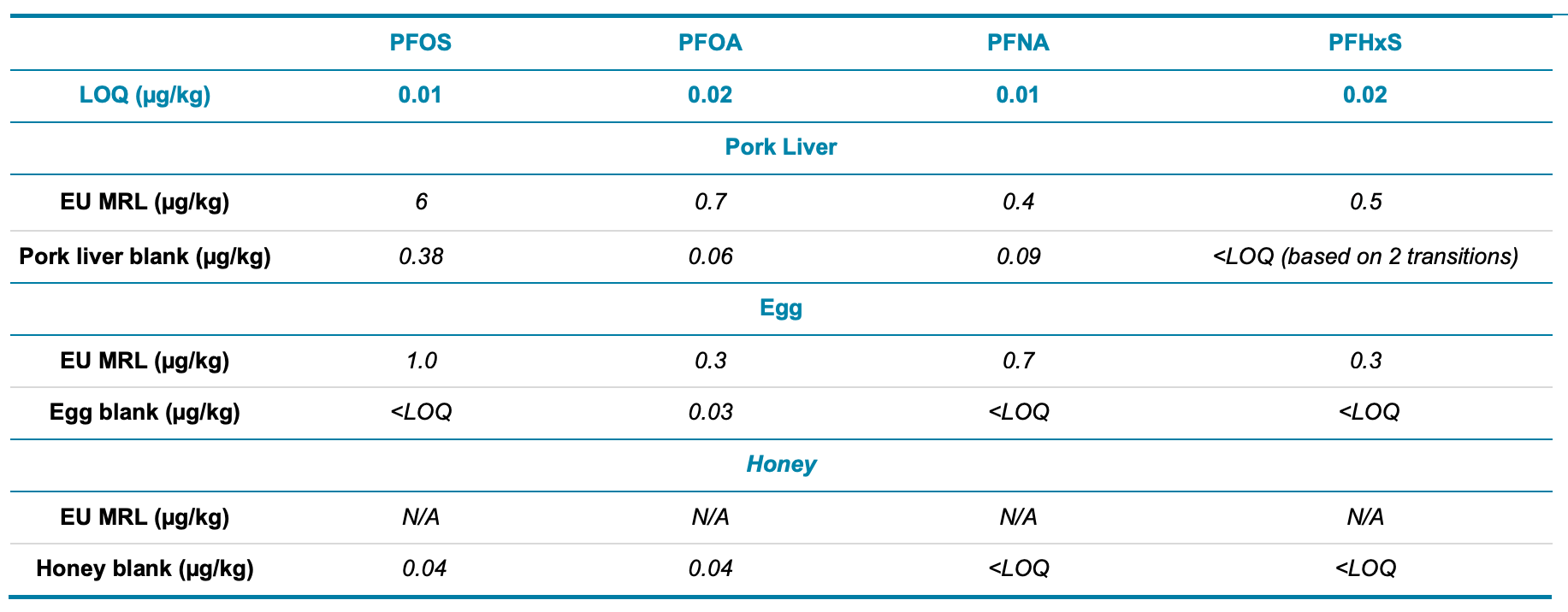 Click to enlarge
Click to enlarge