Targeted quantitation of milk and egg allergens in vegan foods
Sabarinathan1, Lakshmanan Deenadayalan1, Sashank Pillai1, Holly Lee2 and Craig M. Butt3
1SCIEX, India; 2SCIEX, Canada; 3SCIEX, USA
Abstract
This technical note demonstrates a sample preparation and LC-MS/MS method for the determination of milk and egg allergens in vegan and conventional foods. The SCIEX 7500 system achieved limits of quantitation (LOQs) in the range of 0.1–10 mg allergen/kg food, which provided sufficient sensitivity to comply with the recommended levels described in the AOAC standard method performance requirement (SMPR) and by the Voluntary Incidental Trace Allergen Labeling (VITAL) program for allergen labeling. Signature peptides of α-casein, β-lactoglobulin and ovalbumin were quantified with matrix-matched calibration standards. Both standards and spikes were incurred with allergen reference materials prior to sample preparation to assess the impact of protein modifications and interactions with other food ingredients on method performance. The developed method successfully detected the presence of milk and egg allergens in a variety of locally purchased vegan and conventional foods.
Key benefits of the SCIEX 7500 system for food allergen quantitation
- Method sensitivity: The LOQs of all allergen peptides in matrix met the recommended sensitivity levels provided by the VITAL thresholds and the AOAC SMPR for food allergens.
- Quantitation with matrix-matched standards: Good accuracies (±30%), precision (%CV <30%), retention time (RT, <5%) and ion ratio (±30%) tolerances were achieved for incurred standards and matrix spikes at LOQ and higher levels.
- Method evaluation under real-world conditions: Method performance was assessed by matrix spikes incurred with allergen reference materials prior to sample preparation and heating.
- Allergen screening in locally purchased foods: The sensitivity of the SCIEX 7500 system enabled the detection of undeclared milk and egg allergens in vegan products.
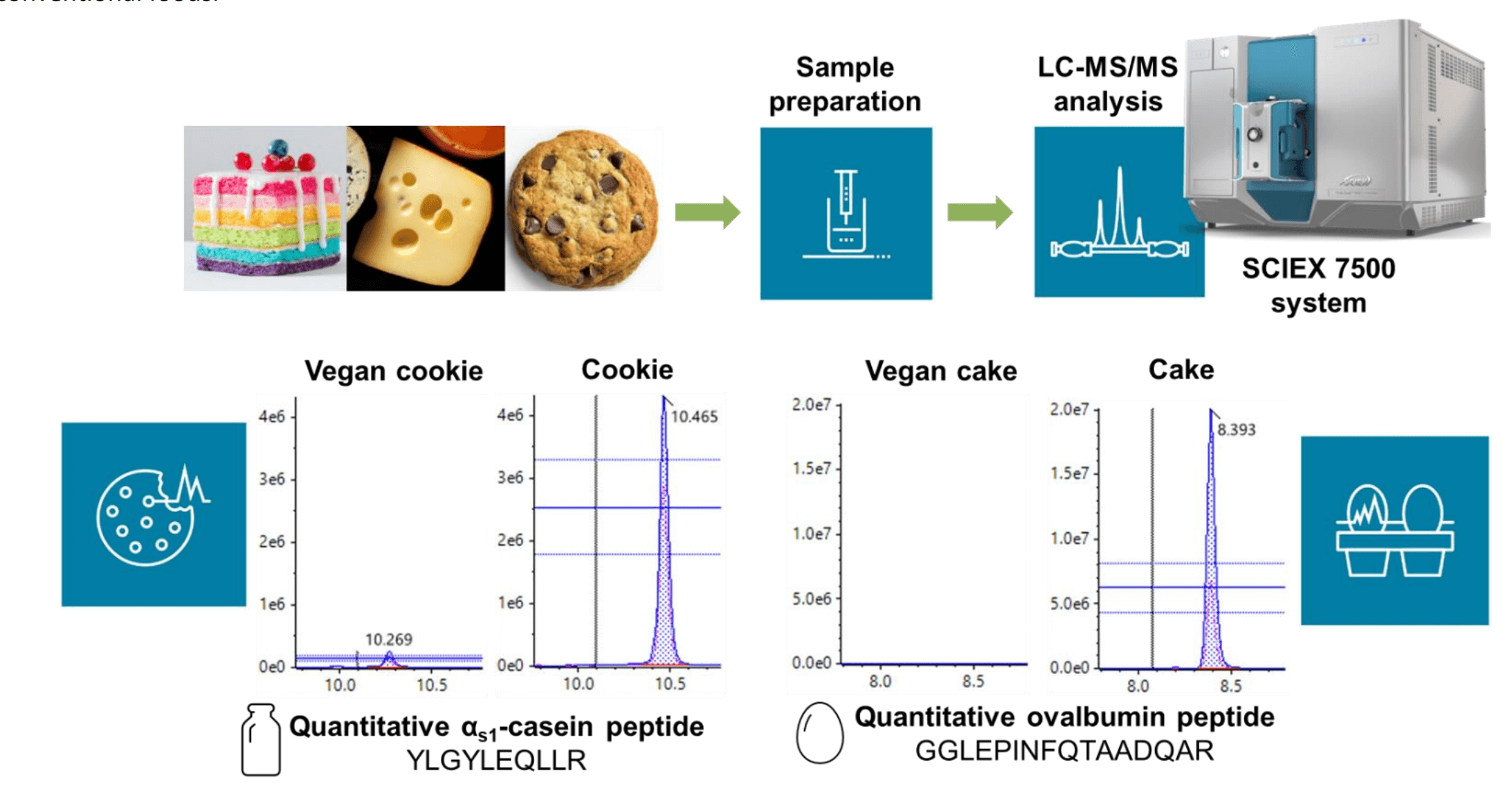
Figure 1. Workflow for the simultaneous detection of milk and egg allergens in cookie, cheese and cake samples on the SCIEX 7500 system. Representative extracted ion chromatograms (XICs) are shown for the quantitative peptides of milk αS1-casein YLGYLEQLLR and egg ovalbumin GGLEPINFQTAADQAR monitored and in some cases, detected in both vegan and conventional cookie and cake samples.
Introduction
Due to the prevalence of food allergies (2.4% of children and 1.6% of adults) reported worldwide,1 many countries have mandated allergen labeling to ensure food safety. Milk, egg, fish, crustacean, shellfish, tree nuts, peanut, wheat and soy are commonly identified as the major food allergens, although some regions have included additional allergens such as celery, mustard and lupin in the European Union (EU)2 and sesame in the US.3 However, the presence of undeclared allergens can occur from cross-contamination during food processing, which is often mitigated by including a precautionary statement such as “may contain” product labels.4 Although allergen labeling is regulated, product claims of “vegan” or “plant-based” are not and are used to advise dietary preferences. Traces of egg and milk have been previously reported in vegan and plant-based foods,5,6 although most consumers believe these products to be free of animal-based ingredients.5,7 This confusion can lead to unexpected allergic reactions. Even trace levels (mg/kg or ppm) can translate into exposure doses exceeding the FAO/WHO reference dose (RfD) thresholds for triggering allergic reactions.8 The Australia-New Zealand Allergen Bureau adopted these RfDs to create action levels for allergen labeling as part of their Voluntary Incidental Trace Allergen Labeling (VITAL) program. While not regulated, these levels help guide the method sensitivity required for food allergen quantitation.
Enzyme-linked immunosorbent assays (ELISAs) are commonly used for routine food allergen quantitation, but this technique suffers from cross-reactivity, test variability between suppliers and is typically limited to only single-allergen detection. In contrast, targeted LC-MS/MS assays are capable of multi-allergen detection with high sensitivity and possess the selectivity required for challenging food matrices. In this technical note, a combined sample preparation and LC-MS/MS method was developed for the simultaneous detection and quantitation of milk and egg allergen peptides in both vegan and non-vegan food products. Here, various vegan products were incurred with egg and milk allergen reference materials to assess method performance in test matrices that are representative of commercially available food products.
Methods
Reagents: The reagents used for the protein extraction and digestion are listed elsewhere.9 All allergen reference materials were purchased from Sigma Aldrich and included lyophilized powders of α-casein (casein marker) and β-lactoglobulin (whey marker) from bovine milk and ovalbumin from chicken egg white. A stock solution of 2 mg/mL of α-casein, 5 mg/mL of β-lactoglobulin and 5 mg/mL of ovalbumin was prepared by solubilizing the powders in water with 0.5% (v/v) formic acid for spiking.
Food samples: Both vegan and non-vegan food products, including cookie mix, cake mix, pancake mix, cookies, cakes and cheese, were purchased from a local supermarket. A subset of the vegan products was pre-screened to determine which samples were allergen-free for subsequent method development.
Preparation of incurred matrix-matched standards in raw vegan foods: Matrix-matched standards with final concentrations ranging from 0.1 to 100 mg allergen/kg food were prepared by fortifying 1 g each of blank cookie mix, cheese and cake mix with the stock reference material mix. Quality control (QC) samples with final concentrations of 10 mg/kg and 50 mg/kg were prepared in the same manner to determine apparent recoveries.
Preparation of incurred vegan foods prior to baking: Prior to spiking, the cookie and cake mixes were combined with oil and water (25 g of cookie mix with 12 g of oil; 20 g of cake mix with 10 g of water and 6 of oil). For cheese, 5 g was used for spiking. Incurred levels ranging from 1 to 500 mg allergen/kg food were prepared by fortifying the blank food with varying amounts of the stock reference material mix. Each incurred sample was baked for 25–35 min at 150–180°C in a conventional oven.
Preparation of pre- and post-spiked food matrices to determine absolute extraction recoveries: The workflow for preparing pre-and post-spiked food digests (n = 3, each) to assess extraction recoveries is described in Figure 2. For the pre-spikes, the blank food was fortified with the stock allergen mix before sample preparation. For the post-spikes, the blank food and the allergen mix underwent separate sample preparation before they were combined. This ensures the recoveries are calculated based on allergen peptides that had been extracted and digested in both the pre- and post-spikes. Absolute recoveries were calculated based on the quotient of the average peak areas in the pre-spiked to the post-spiked digests.
Figure 2. Preparation of pre- and post-spiked matrix for the determination of absolute or extraction recoveries. Extraction recovery was determined at 10 mg allergen/kg food based on the quotient of the peak areas in the pre-spiked food digest (A) and the blank food digest post-spiked with the tryptic digest containing the peptides from the allergen reference materials that underwent the same sample preparation (B).
Lipid removal, protein extraction, digestion and clean-up: All samples (matrix-matched standards, QCs, matrix spikes and real-world unknowns) were subjected to the following sample preparation. Briefly, 1 g of food was defatted with 2 x 5 mL hexane, followed by centrifugation at 4,000 g for 20 min and the supernatant was discarded. After drying under nitrogen gas, the residue was combined with 3.8 mL of extraction buffer (50mM Trizma base and 2M urea) and 200 µL of denaturant (20% w/v, octyl β-D-glucopyranoside) and vortexed at 2,500 rpm for 50 min. An 1.2 mL aliquot of the sample was further centrifuged at 16,000 g for 15 min. 500 µL of the supernatant was then incubated with 50 µL of the reducing agent (50mM Tris-(2- carboxyethyl)-phosphine) at 60oC for 1 hour to reduce the protein disulfide bonds. After cooling to room temperature, the sample was incubated with 25 µL of the cysteine-blocking agent (100mM S-methyl methane-thiosulfonate) for 20 min. Protein digestion occurred by incubating the sample overnight with 425 µL of digestion buffer (5mM calcium chloride in 100mM ammonium bicarbonate) and 20 µL of 1 µg/µL trypsin. Digestion was quenched by the addition of 30 µL of formic acid. After centrifuging at 16,000 g for 10 min, 500 µL of the supernatant was filtered using a 10 kDa MWCO filter, followed by centrifuging for 15 min. Finally, 150 µL of the filtrate was used for LC-MS/MS analysis.
Chromatography: Chromatographic separation was performed on an ExionLC AD system using a Kinetex C18 column (100 x 3.0 mm, 2.6 µm, Phenomenex P/N 00D-4462-Y0). Table 1 lists the gradient conditions used with an injection volume of 2 µL and a column temperature of 30°C.
Table 1: Chromatographic gradient for the analysis of milk and egg allergens.
Mass spectrometry: Analysis was performed using scheduled multiple reaction monitoring (sMRM) in positive electrospray ionization mode on the SCIEX 7500 system. Table 2 lists the source and gas parameters used. Table 3 lists the optimized MRM parameters for the target egg and milk allergen peptides.
Table 2. Source parameters for allergen detection on the SCIEX 7500 system.
Data processing: Data acquisition and processing were performed using the SCIEX OS software, version 3.3.1.
Table 3. MRM transitions for the target protein and peptides of the milk and egg allergens with their retention times (RTs), MRM transitions and optimized compound-dependent parameters. The first listed transition for each peptide was used for quantitation, while the second transition was used for confirmation.
Chromatographic separation of marker peptides for milk and egg allergens
The current proteotypic peptides used here were selected based on previous discovery proteomics work that involved rigorous assessment of their uniqueness to each allergen and robustness to post-translational modifications from food processing.10 Milk allergen detection relied on monitoring for α-casein and β-lactoglobulin, which represent the casein and whey protein fractions of milk. Ovalbumin was the only egg marker protein monitored here; therefore, this method can only be used to screen for foods containing egg white as an ingredient. To increase the confidence of allergen detection, 2 peptides per protein and 2 MRM transitions per peptide were monitored for each allergen. Chromatographic optimization enabled good separation of the 6 target peptides in both solvent and food matrices (Figure 3).
Figure 3. Chromatographic separation of target peptides of egg and milk allergens in matrix-matched calibration standards at 100 mg/kg. Representative extracted ion chromatograms (XICs) are displayed for the 6 target peptides in raw vegan cookie dough, cheese and cake mix incurred at 100 mg allergen/kg food.
Linear performance and sensitivity of the SCIEX 7500 system in matrix-matched standards
LC-MS/MS-based quantitation of food allergens is challenged by the lack of harmonization and sometimes even lack of transparency in calibration strategies, as evidenced by the diversity of approaches reported in the literature (Figure 4).9,11,12 As a result, quantitative performance can vary depending on the choice of calibrant and matrix, the quantitation technique, the fortification step and calibration sample preparation. Table 4 presents the linear performance of the matrix-matched calibration curves produced in cookie dough, cheese and cake mix that had been incurred with varying levels of milk and egg allergen reference materials before undergoing sample processing. Both r and r2 values were included here as these values are often inconsistently reported in the literature. Most of the peptides exhibited r-values >0.98 and r2 -values >0.96, consistent with previous studies using a similar matrix-matched external calibration strategy.11,12 Linearity can be improved by using isotope-labeled proteins or peptides to correct for sample preparation losses, matrix effects and instrument variability, but these were not used here due to cost and availability issues.
Figure 4. An overview of different food allergen calibration strategies for food allergen quantitation. While not exhaustive, these approaches represent those most commonly reported in the literature. The options used in this work are highlighted in green.
Table 4. Quantitative performance of matrix-matched calibration curves in raw vegan cookie dough, cheese and cake mix. All target peptides are listed with their dynamic range, correlation coefficient (r), coefficient of determination (r 2 ), accuracy and precision at the LLOQ and other levels.
The sensitivity of the SCIEX 7500 system enabled accurate (±30%) and reproducible (%CV <30%) quantitation at LOQs ranging from 0.1 to 10 mg allergen/kg food in the vegan cookie dough, cheese and cake mix (Table 4). The representative XICs in Figure 5 showed strong responses (S/N >10) and good peak shapes for the quantitative peptide markers of each allergen at the LOQ level in the incurred matrix. LOQ selection was based on the quantitative peptide markers meeting the acceptance criteria of RT error (<5%), accuracy (±30%), precision (%CV <30%), ion ratio (±30%), S/N (>10 for the quantifier transition and >3 for the qualifier transition) and confirmatory peptide transitions with S/N >3. Although the instrument sensitivity enabled the detection of the αS1-casein peptide YLGYLEQLLR as low as 0.1 mg/kg, a higher LOQ of 2 mg/kg was selected to compensate for its background presence in the blank vegan cheese (Figure 5).
Based on clinical studies, the VITAL system established allergen RfDs, reported as total protein in mg, to calculate the action levels that would trigger precautionary labeling.8 As these RfD thresholds are often used to evaluate method sensitivity, the LOQs reported in Table 4 as mg allergenic ingredient/kg food were converted to mg protein/kg food using milk and egg protein composition abundances previously reported in the literature (Table 5). The converted LOQs from this study were below the VITAL action levels calculated based on allergen RfDs and food serving sizes (Table 5), demonstrating the sensitivity of the SCIEX 7500 system.
Figure 5. Representative XICs of the quantitative peptide marker of each target milk and egg allergen in raw vegan cookie dough, cheese and cake mix. Each row shows the XICs for the quantifier (blue trace) and qualifier (pink trace) transitions with ion ratio tolerance lines in the blank food matrix and incurred food matrix at the LOQ. The selection of LOQ was based on the quantitative peptide marker meeting the acceptance criteria of RT error (<5%), accuracy (±30%), precision (%CV <30%), ion ratio (±30%) and signal-to-noise ratios (S/N >10 for the quantifier and S/N >3 for the qualifier). The confirmatory peptides (not shown here) were also monitored to ensure their S/N were >3 for positive detection.
Table 5: Converted LOQs (mg protein/kg food) for comparison against VITAL action levels (mg protein/kg food) calculated based on food serving sizes.
Figure 6 shows how these complex calculations can be directly performed and presented in the results table using the custom calculations and conditional lookup features in the Analytics module of the SCIEX OS software. Using the most updated VITAL 4.0 RfD of 2 mg total protein for both milk and egg,8 action levels of 92 mg protein/kg for cookie mix, 20 mg protein/kg for cheese and 77 mg protein/kg for cake mix were obtained for each allergen based on the serving size (g) recommended on the label of each vegan product tested.
Figure 6. Custom calculations and conditional lookup tables in SCIEX OS software. The conditional lookup tables in the SCIEX OS software enable the user to perform complex calculations, such as the LOQ conversion from mg allergenic ingredient/kg food to mg proteins/kg food and VITAL-based action levels, directly in the results table of the software without having to export the data to third-party software, minimizing the risk of error and the need for revalidation.
Quantitative performance in matrix spikes
Apparent recovery accounts for matrix effects and extraction losses based on comparing the calibration-derived value against the known fortified value. At the incurred levels at the LOQ of each quantitative peptide (0.5–5 mg/kg) and at 2 higher QC levels of 10 and 50 mg allergen/kg food, apparent recoveries of 75–128% were achieved (Table 6). This affirms the use of matrix-matched standards to correct for sample preparation losses and matrix effects. In contrast, absolute recovery measures the extraction yield based on comparing the amounts recovered in the pre- and post-spiked samples. Absolute recovery ranges of 25–57% and 51–86% were achieved in cookie mix and cheese, respectively, while <20% recoveries were obtained in the cake mix. Despite these lower recoveries, they are consistent with those previously reported (20–60%) in the literature13-15 and provide realistic insights on how incurred allergens are lost during the overall extraction and digestion process. Matrix-matched calibration with isotope-labeled internal standards is currently considered to be the “gold standard” approach for food allergen quantitation,9-11,15,16 but is also the least adopted by routine laboratories due to the high cost and time associated with preparing and procuring these standards.
Table 6. Apparent and absolute recoveries calculated based on the quantifier transition of each allergen peptide marker in vegan cookie dough, cheese and cake mix. Apparent recovery compares the amount of analyte recovered measured from a calibration curve against the known fortified value, while absolute recovery refers to the extraction yield based on comparing the amounts recovered in the pre- and post-spiked samples.
Comparison with AOAC SMPR 2016.002
Overall, the method performance in the 3 food matrices tested was within the acceptance criteria outlined in the AOAC SMPR 2016.002 for food allergen quantitation (Table 7).17 The sensitivity of the SCIEX 7500 system enabled starting at a lower analytical range than that defined in the SMPR. All allergen peptides exhibited LOQs in the range of 0.5–5 mg allergen/kg food, meeting the SMPR method quantitation limit (MQL) of 5 and 10 mg allergen/kg food for egg and milk, respectively. While these LOQs were not calculated based on the statistical approach prescribed in the AOAC SMPR, they were experimentally derived from incurred matrices taken through the entire sample preparation process and selected based on RT, accuracy, precision, ion ratio and S/N. Apparent recoveries ranged from 66 to 128% at the LOQ and the two higher QC levels. These apparent recoveries were largely within the SMPR recommended recovery range of 60–120%. The method also demonstrated excellent repeatability (RSDr) of <20% based on 3 independent replicates across all 3 incurred levels in the food matrices.
Table 7. Method performance comparison against the AOAC SMPR 2016.002 for food allergen quantitation.
Allergen detection in real-world market samples
The method applicability was tested on locally purchased food products, which included both conventional and vegan or plant-based products. Quantitation was not performed here due to the lack of internal standards, while individual preparation of matrix-matched standards for each sample was impractical and labor-intensive. Instead, allergen screening was performed based on the detection of peptide markers meeting the previously described acceptance criteria of peak area reproducibility, RT error, ion ratio and S/N. Table 8 lists the detection results in the vegan and non-vegan foods screened, with example XICs for the quantitative peptides of milk αS1-casein and egg ovalbumin monitored in cheese and cake samples. Most of the food labels accurately reflected the expected presence of milk and egg allergens, but several samples exhibited positive detection despite the undeclared allergens in the ingredients list. For example, no allergens were listed on the vegan cheese label, but the SCIEX 7500 system detected both the αS1-casein and β-lactoglobulin peptides. However, positive detection would only trigger allergen labeling if the measured concentration exceeds the VITAL action level. At levels below the VITAL thresholds, the trace presence of allergens is considered safe for 95–99% of the allergic population.8
Table 8. Detection of milk and egg allergen peptide markers in vegan and non-vegan foods purchased locally. Positive detection (denoted by “Y”) is based on the acceptance criteria of peak area reproducibility (%CV <30%), RT error (<5%), ion ratio (±30%), and S/N (>10 for the quantifier transition and >3 for the qualifier transition for the quantitative peptide marker; >3 for both transitions for the confirmatory peptide marker). Non-detection is denoted by “N”. Foods with allergens listed on the label are denoted with “Y”, where the superscripts “M” and “E” refer to milk and egg ingredients, respectively. Representative XICs of the cheese and cake samples highlighted in yellow and pink are displayed below.
Conclusions
- The SCIEX 7500 system achieved LOQs of 0.5–5 mg allergen/kg food for milk and egg allergen peptides in cookie, cheese and cake samples
- These LOQs enabled the detection of milk and egg allergens at levels below the recommended sensitivity required for complying with the VITAL thresholds and AOAC SMPR.
- Method performance was assessed by incorporating real-world challenges with food allergen quantitation, such as matrix spikes incurred prior to sample processing and the impact of heat on peptide detectability in baked foods.
- SCIEX OS software provided user-friendly tools, such as conditional lookup tables, to facilitate complex calculations of protein-based LOQs and VITAL action levels for allergen labeling.
- Method application to commercial foods successfully identified both declared and undeclared allergens in the ingredients label.
References
- Bartha, I.; Almulhem, N.; Santos, A.F. Feast for thought: A comprehensive review of food allergy 2021-2023. J. Allergy Clin. Immunol. 2024, 153, 576-594.
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers. Official Journal of the European Union L 304. November 2011, pp. 18-63.
- H.R. 1202 FASTER Act of 2021. 117th Congress.
- Allen, K.J. et al. Precautionary labelling of foods for allergen content: are we ready for a global framework? World Allergy Organ J. 2014, 7, 10.
- Dominguez, S.; Théolier, J.; Lizée, K.; Povolo, B.; Gerdts, J.; Godefroy, S.B. “Vegan” and “plant-based” claims: risk implications for milk- and egg-allergic consumers in Canada. Allergy Asthma Clin. Immunol. 2023, 19, 74.
- Marsh, S. UK vegan products found to contain milk or egg. The Guardian. Published July 2023.
- Chartered Trading Standards Institute. Vegan and plant-based foods. Published July 2023.
- FAO/WHO. Risk assessment of food allergens. Part 2: review and establish threshold levels in foods for the priority allergens: meeting report. Published January 2023.
- New, L.S.; Stahl-Zeng, J.; Schreiber, A.; Cafazzo, M.; Liu, A.; Brunelle, S.; Liu, H-F. Detection and quantitation of selected food allergens by liquid chromatography with tandem mass spectrometry: First action 2017.17. J. AOAC Int. 2020, 103, 570-583.
- New, L.S.; Baghla, R.; Schreiber, A.; Stahl-Zeng, J.; Liu, H-F. Simultaneous analysis of 12 food allergens in baked and raw food products using the LC-MS/MS QTRAP 4500 system. SCIEX technical note, RUO-MKT-02-2846-B.
- Xiong, W.; Parker, C.H.; Boo, C.C.; Fiedler, K.L. Comparison of allergen quantification strategies for egg, milk, and peanut in food using targeted LC-MS. Anal. Bioanal. Chem. 2021, 413, 5755-5766.
- Monaci, L.; De Angelis, E.; Guagnano, R.; Ganci, A.P.; Garaguso, I.; Fiocchi, A.; Pilolli, R. Validation of a MS Based Proteomics Method for Milk and Egg Quantification in Cookies at the Lowest VITAL Levels: An Alternative to the Use of Precautionary Labeling. Foods. 2020, 9, 1489.
- Monaci, L.; Pilolli, R.; De Angelis, E.; Godula, M.; Visconti, A. Multi-allergen detection in food by micro high-performance liquid chromatography coupled to a dual cell linear ion trap mass spectrometry. J. Chrom. A. 2014, 1358, 136-144.
- Lamberti, C.; Acquadro, E.; Corpillo, D.; Giribaldi, M.; Decastelli, L.; Garino, C.; Arlorio, M.; Ricciardi, C.; Cavallarin, L.; Giuffrida, M.G. Validation of a mass spectrometry-based method for milk traces detection in baked food. Food Chem. 2016, 199, 119-127.
- Boo, C.C.; Parker, C.H.; Jackson, L.S. A Targeted LC-MS/MS Method for the Simultaneous Detection and Quantification of Egg, Milk, and Peanut Allergens in Sugar Cookies. J. AOAC Int. 2018, 101, 108-117.
- AOAC International. Standard Method Performance Requirements (SMPRs) for Detection and Quantitation of Selected Food Allergens. AOAC SMPR 2016.002.
 Click to enlarge
Click to enlarge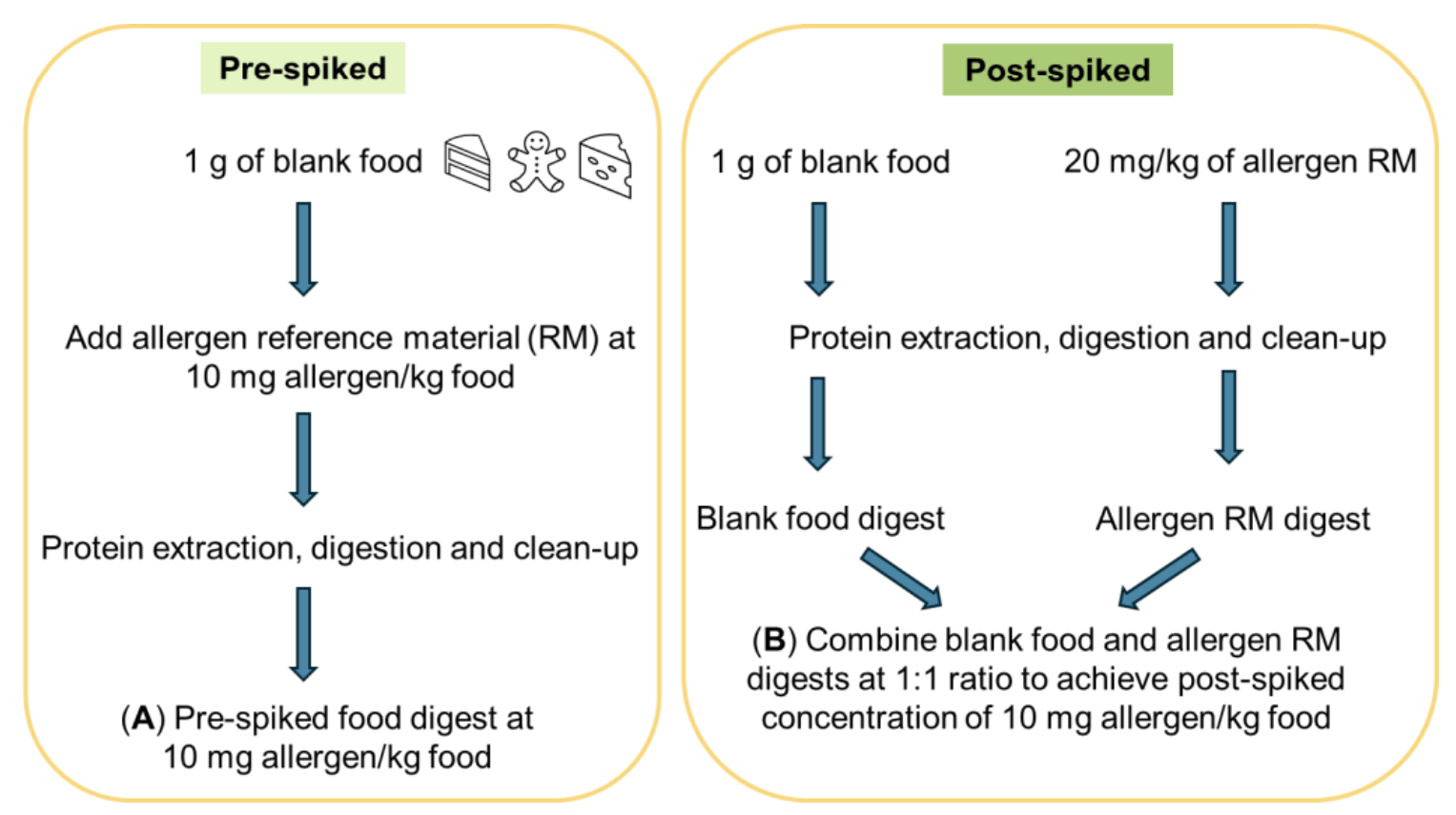 Click to enlarge
Click to enlarge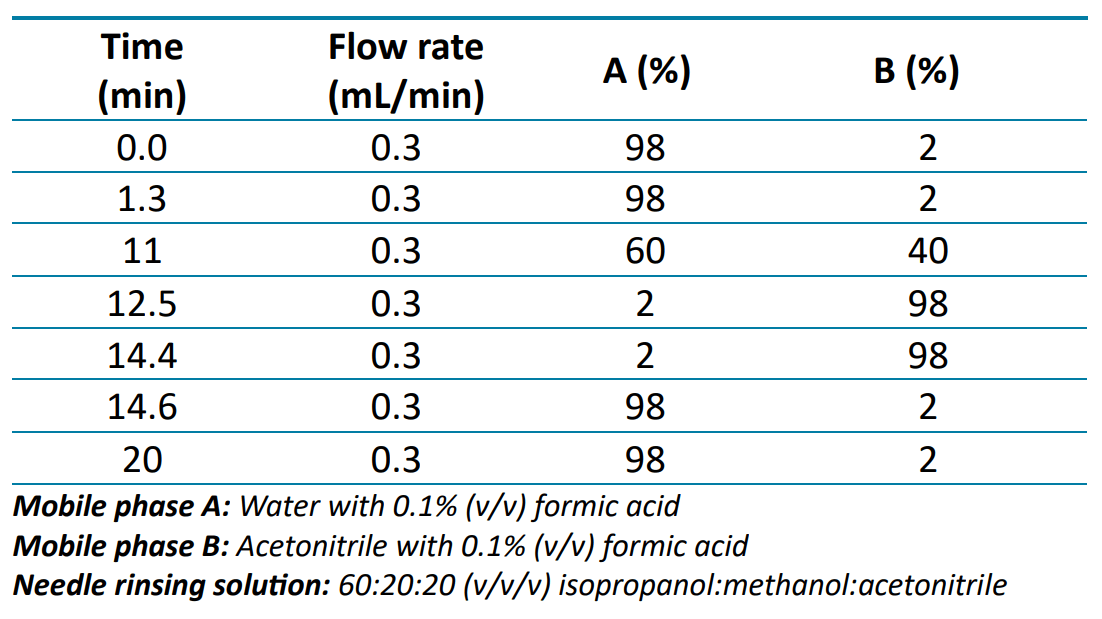 Click to enlarge
Click to enlarge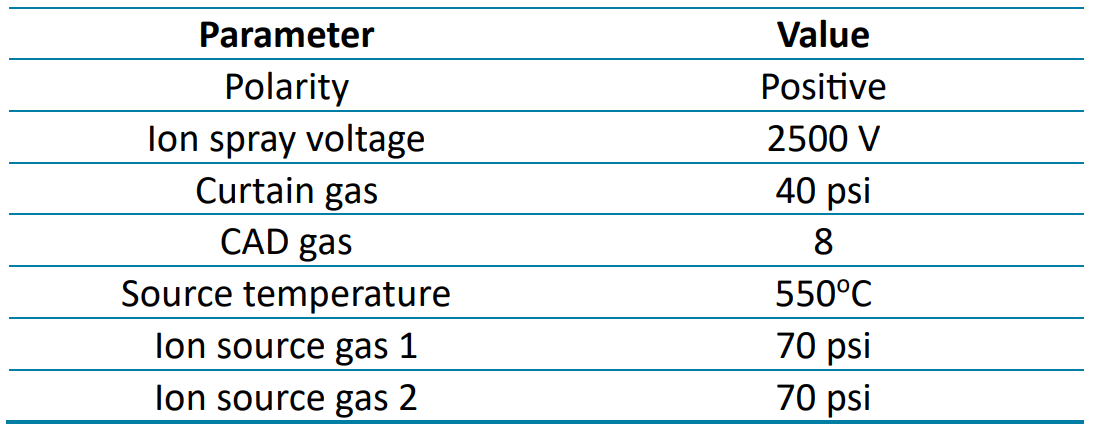 Click to enlarge
Click to enlarge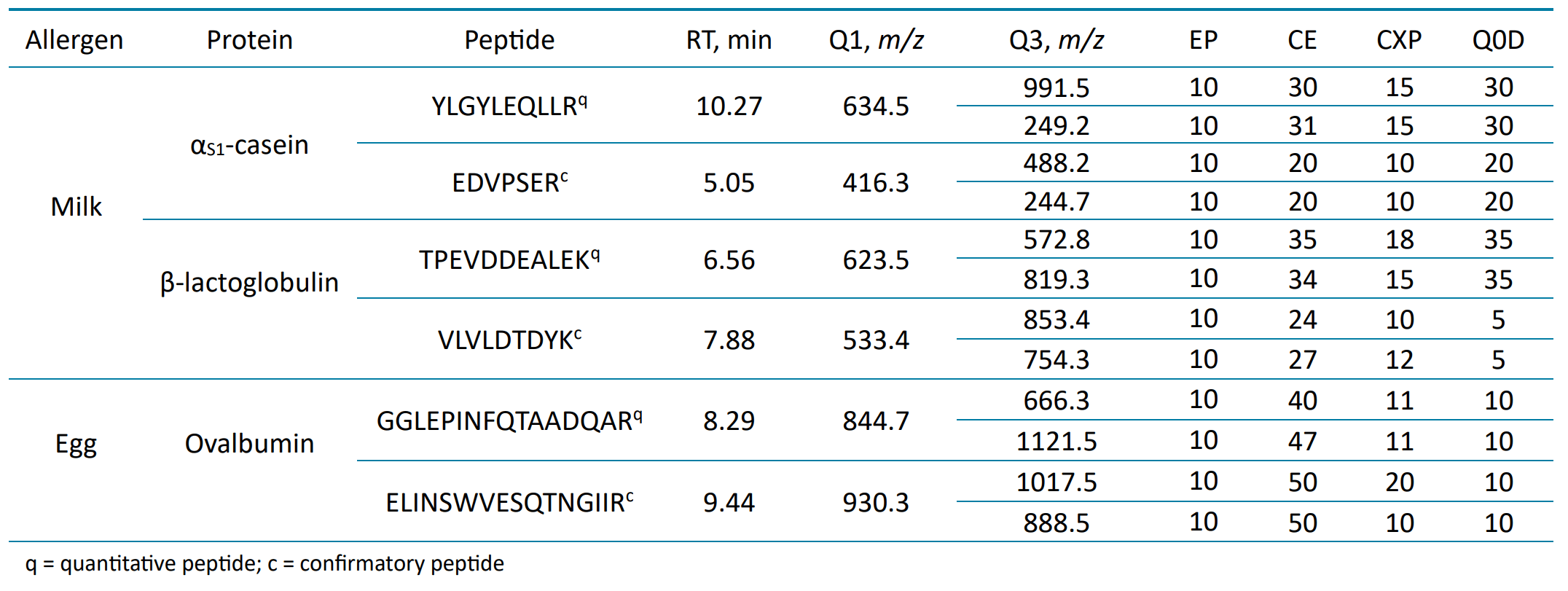 Click to enlarge
Click to enlarge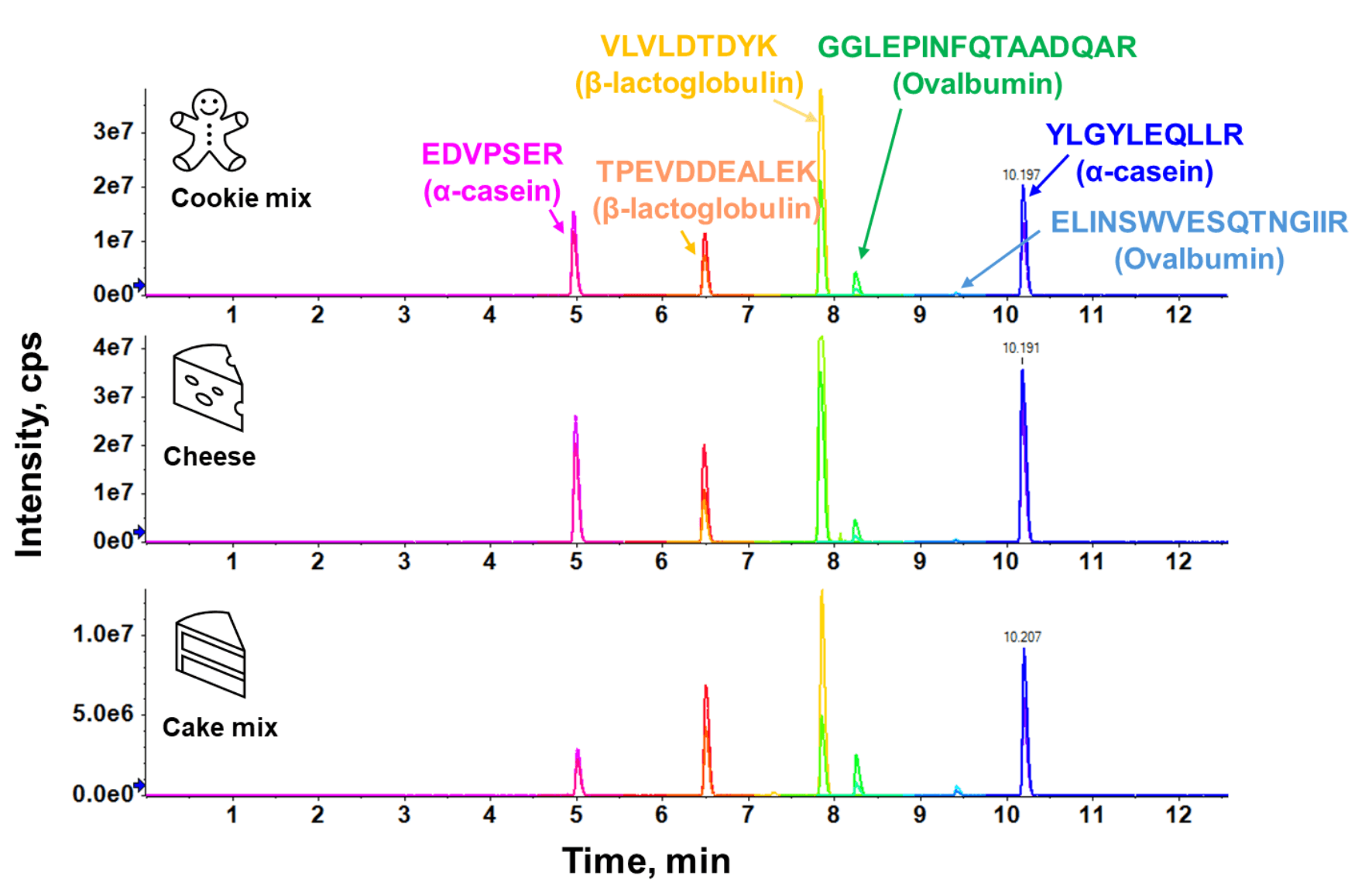 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge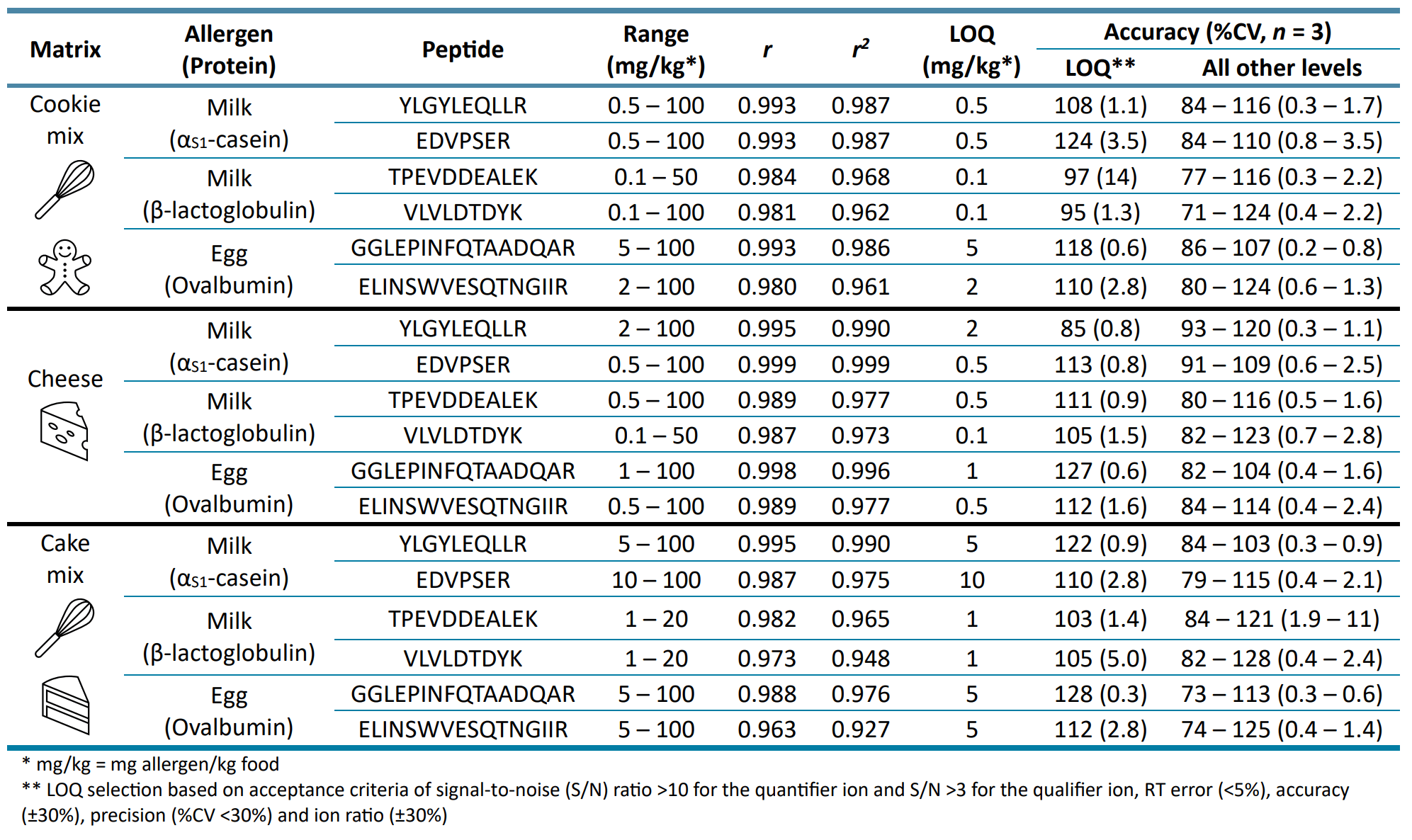 Click to enlarge
Click to enlarge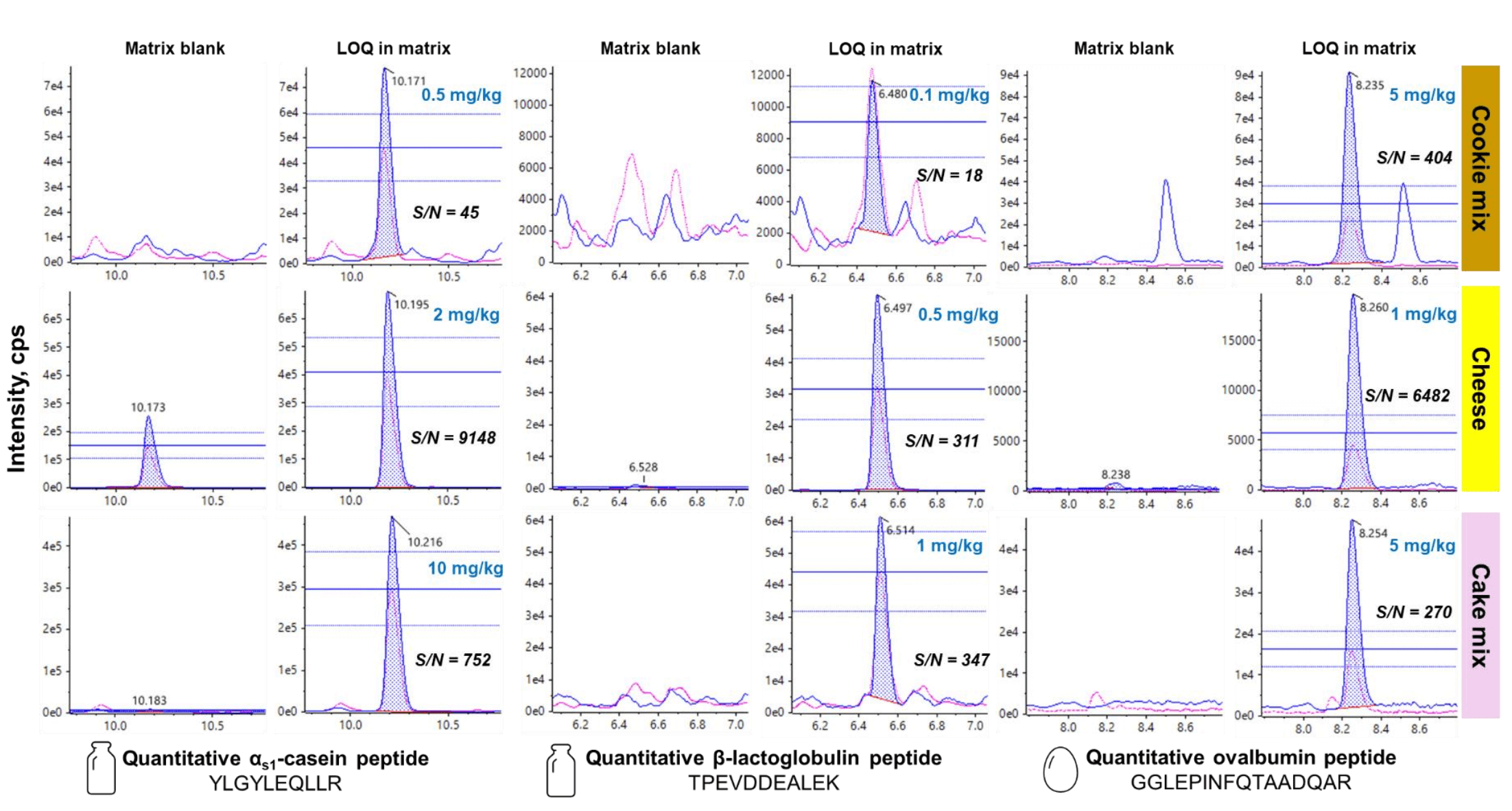 Click to enlarge
Click to enlarge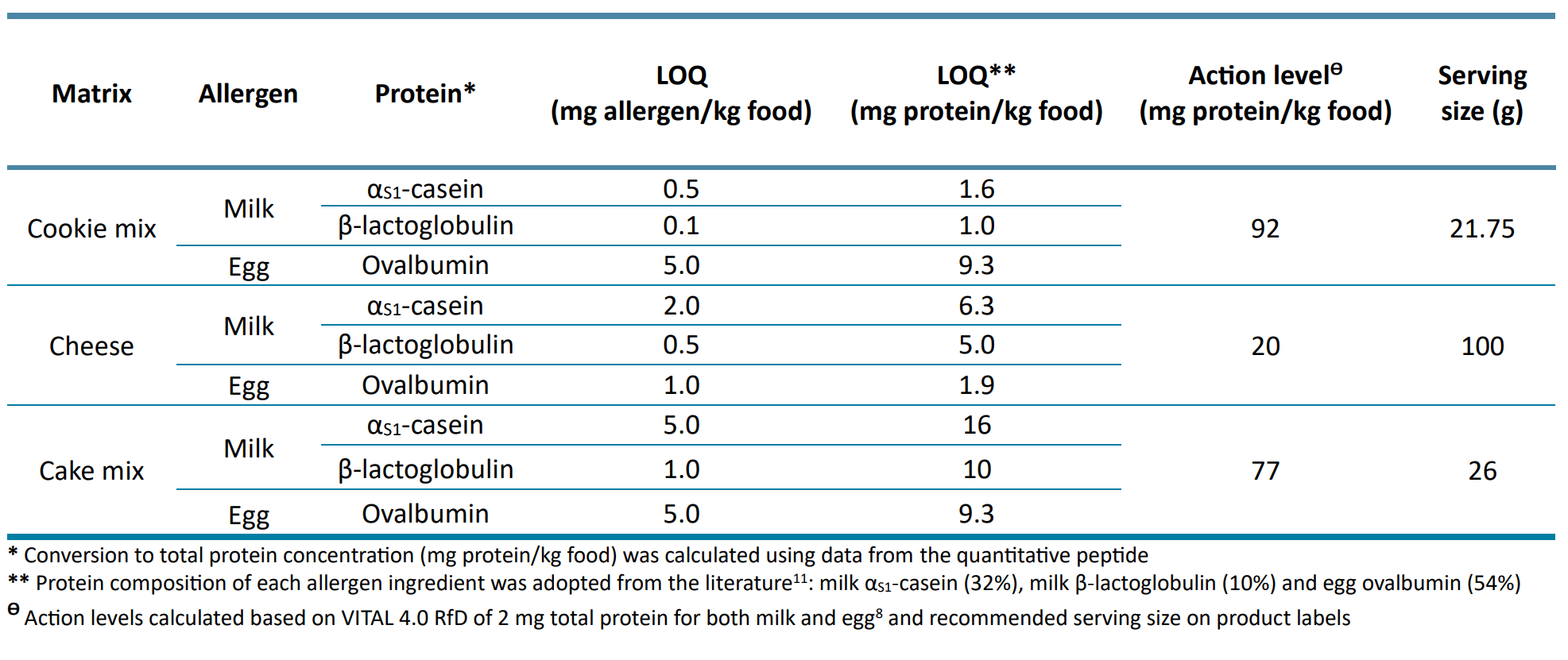 Click to enlarge
Click to enlarge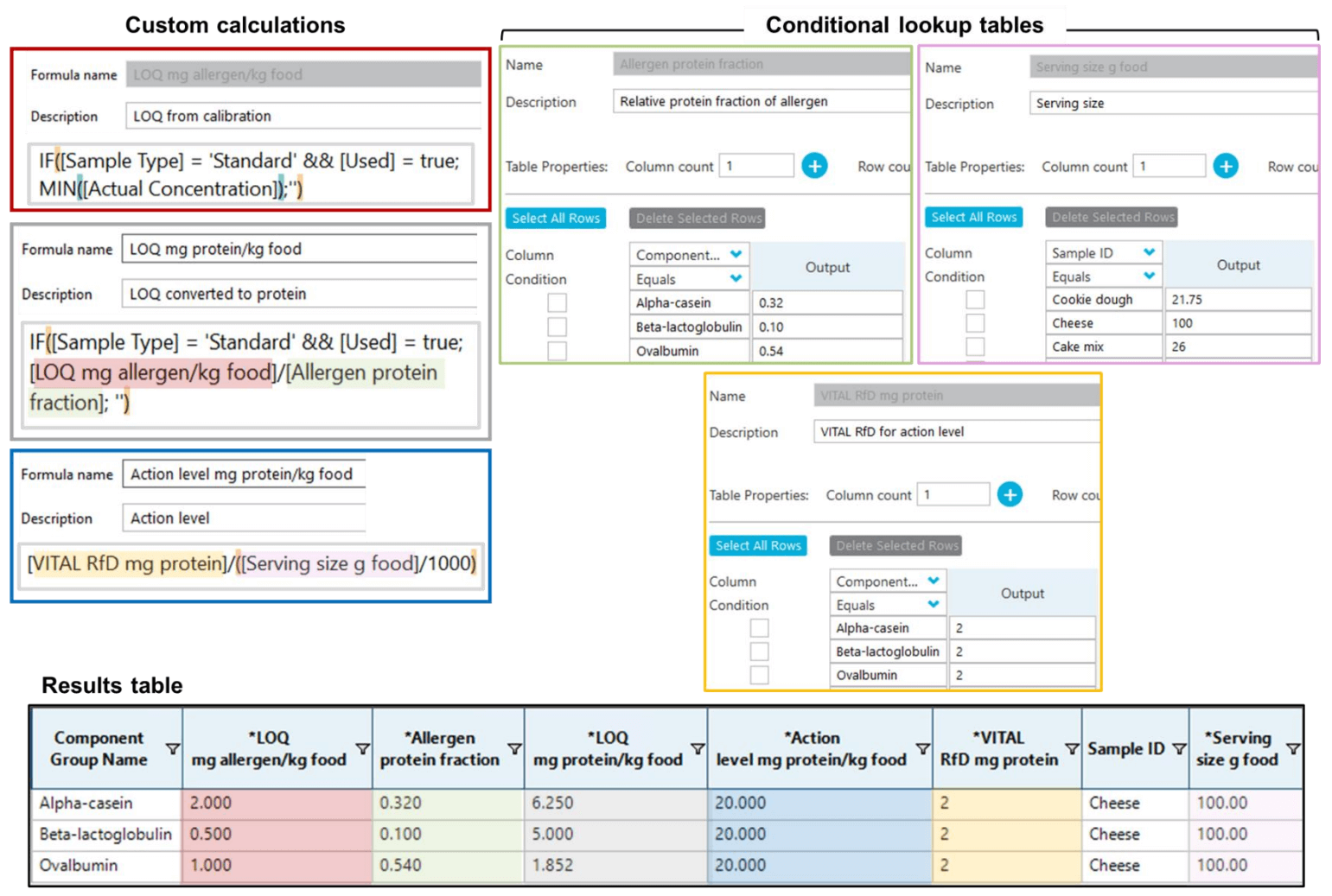 Click to enlarge
Click to enlarge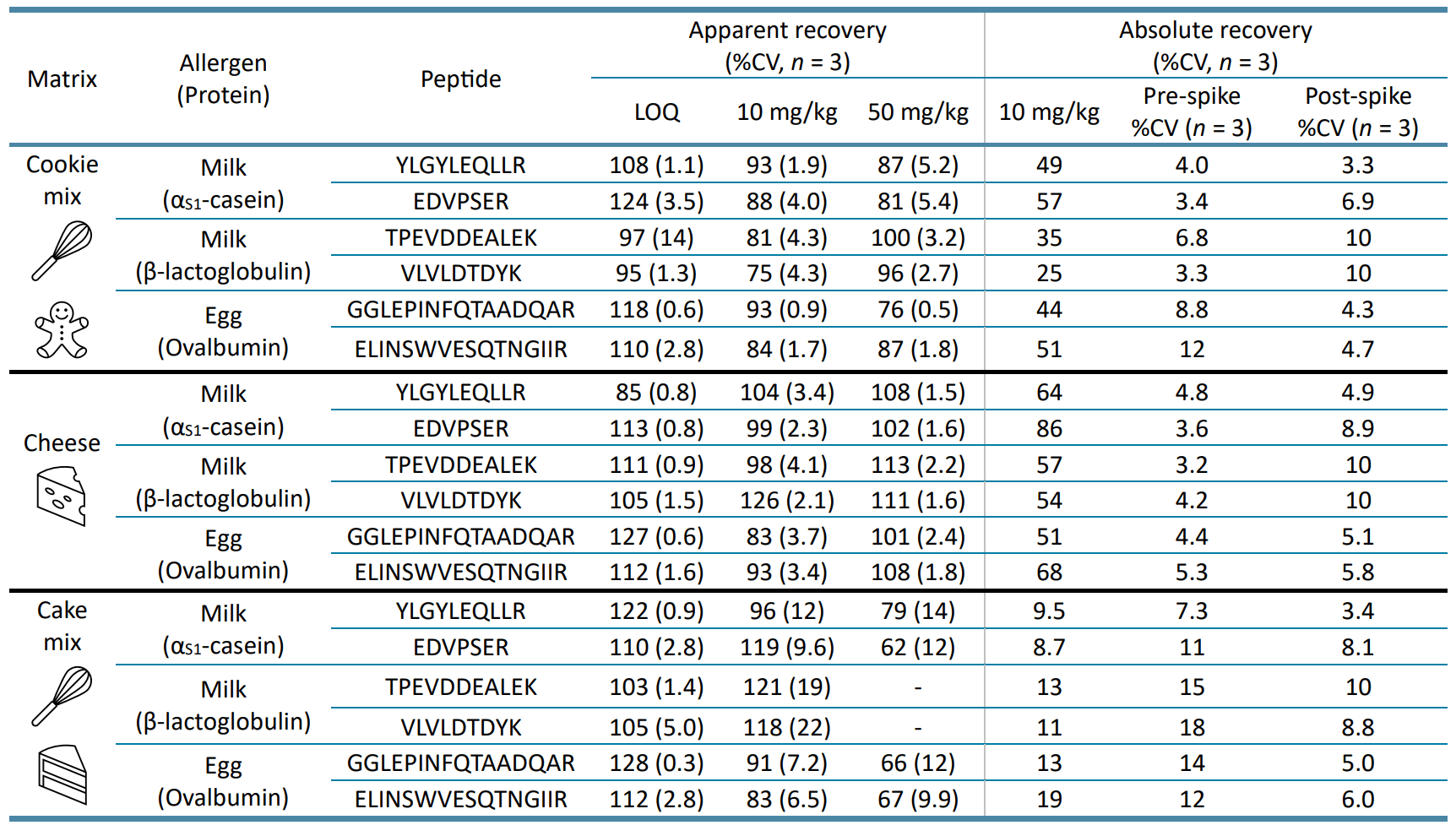 Click to enlarge
Click to enlarge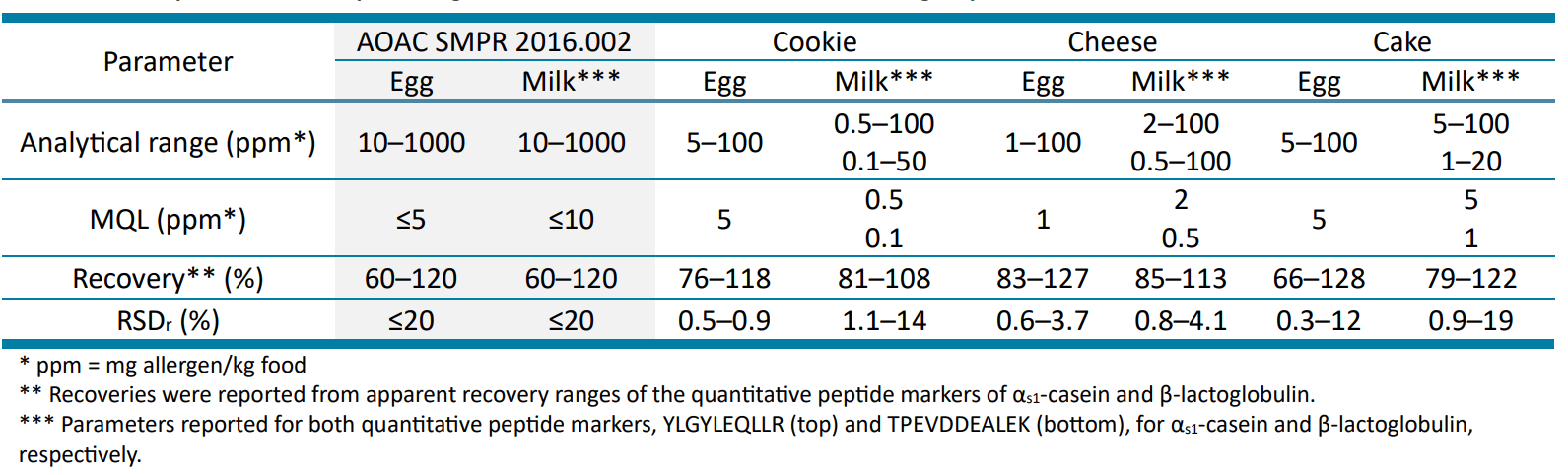 Click to enlarge
Click to enlarge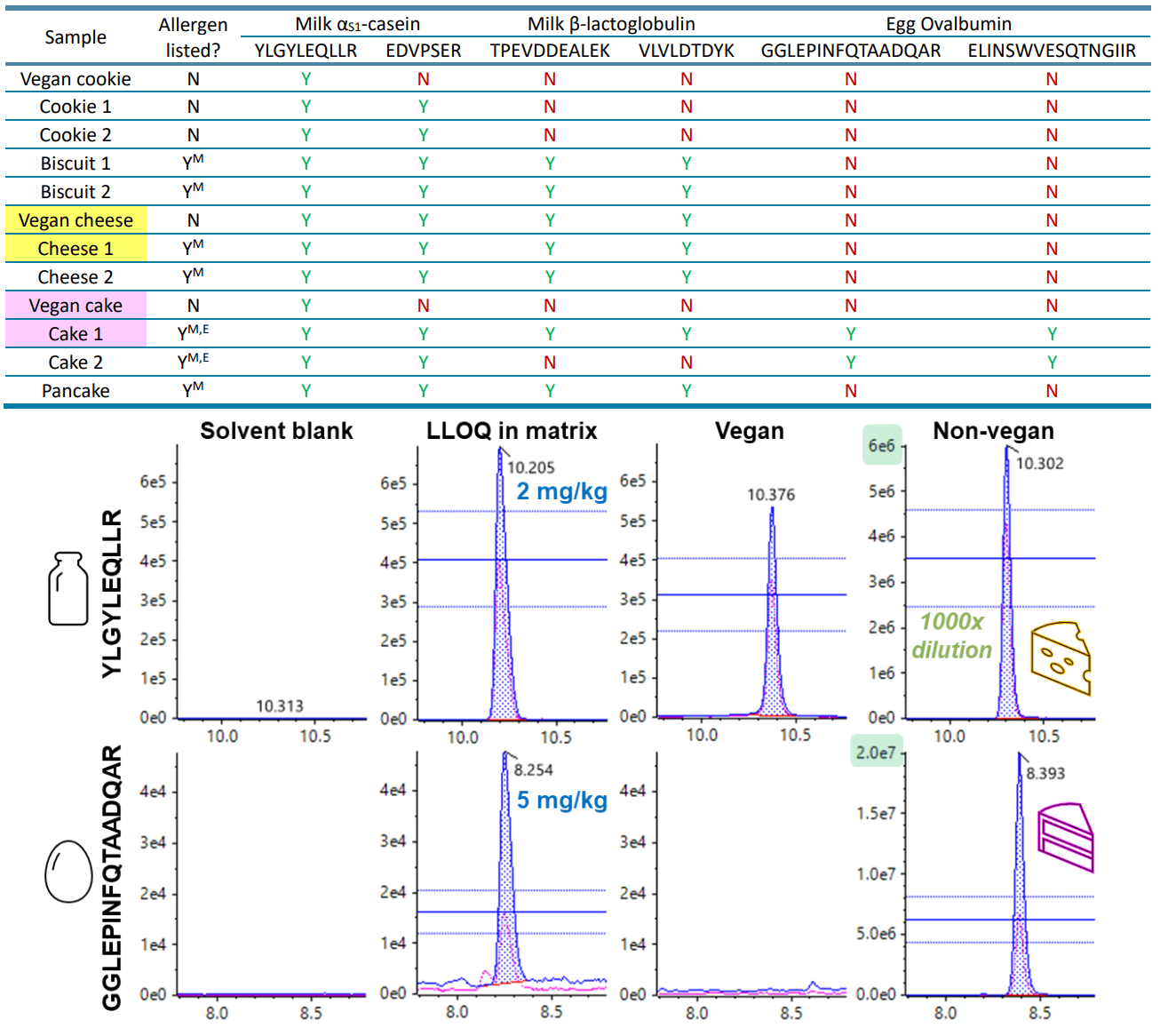 Click to enlarge
Click to enlarge