Fast Forensic Toxicological Screening and Quantitation in Under 3 Minutes
Using SCIEX X500R QTOF System and SCIEX OS Software
Xiang He1 , Adrian M. Taylor2 , Michael Jarvis2 and Alexandre Wang1
1SCIEX, USA; 2SCIEX, Canada
Abstract
There are several critical attributes of a high-confidence and high-efficient forensic screening method: (1) the method must generate all necessary information to unequivocally identify a compound, (2) retrospective analysis can be performed without re-injecting the sample when new targeted compounds are being added to the screened panel, (3) the method should be robust and not require lengthy sample preparation procedures, and (4) data processing should be fast and straight-forward so any laboratory personnel can process data without much difficulty.

Introduction
High resolution accurate mass spectrometry such as time-offlight (TOF) mass spectrometer is a great fit for such screening applications because the data generated from these systems provides structural information for every possible analyte as well as quantitative information. There are mainly two approaches for MS screening purpose: data dependent acquisition (DDA or IDA) and data independent acquisition (DIA). MS is typically coupled with liquid chromatography (LC) for screening applications with runtimes varying from 5 to 20 minutes depending on the study needs.
Forensic toxicological screening is challenging, however, because: (1) usually there are more than hundreds of compounds to be screened with drastically varying chemical properties, (2) new compounds are constantly introduced to evade the current targeted methodologies, (3) current analytical techniques such as immunoassay are slow and inflexible to adapt to the new analytes, lack specificities, and often require multiple individual tests to complete the entire panel of targeted compounds.
In this work, an ultra-fast forensic toxicological screening method has been developed using the SCIEX X500R QTOF system and SCIEX OS Software. The LC runtime of this method is 2.5 minutes. Two different acquisition methods were compared for use in fast screening.
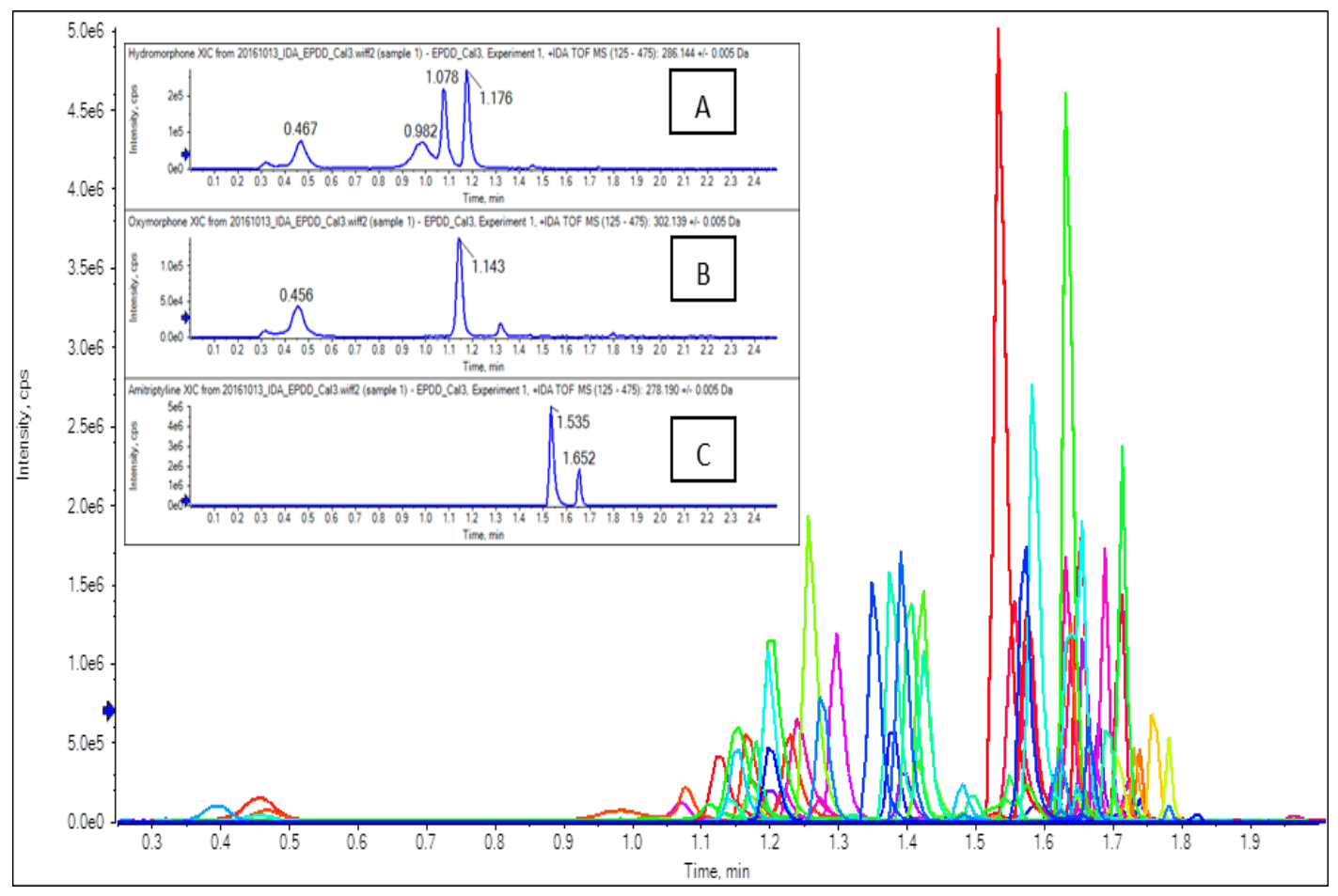
Figure 1. Fast High Resolution Chromatography For Quantitation of Many Designer Drugs. Extracted ion chromatograms (XICs) from TOF MS data of the drugs outlined in Table 1. Even with this fast 2.5-min LC runtime, several groups of isomers were resolved, as shown in the figure insets: (A) norhydrocodone (1.18 min), (B) oxymorphone (0.46 min) and noroxycodone (1.14 min), and (C) amitriptyline (1.54 min) and EDDP (1.65 min).
Methods
Sample Preparation: Dilute and Shoot. Blank urine samples were spiked with multiple drugs commonly found in forensics setting at different concentration levels. Typically, samples were diluted 10-fold in 10% methanol and centrifuged. The clear supernatants were transferred to autosampler vials and 10 μL of each sample was injected.
List of Target Compounds: In this study, we have evaluated two sets of samples with two different groups of compounds for screening. Spiked compounds in Sample Set 1 are shown in Table 1.
Sample Set 1: Urine samples spiked with compounds in Table 1. Four calibrators and two QC samples were prepared. The concentrations in the calibrators were 50%, 100%, 300% and 1000% of cutoff (CO1) concentration. The two QC samples were 200% and 600% of CO1 concentrations respectively. Injection volume was 10 μL.
Sample Set 2: Second set of urine samples contained a calibrator set spiked with compounds similar to what is described in Table 1 with minor differences. Cutoff concentrations in Sample 2 (CO2) were slightly higher than Sample Set 1. There are 7 levels of calibrators: 40%, 80%, 100%, 200%, 300%, 500% and 1000% of CO2 concentration. There were also 20 urine samples with unknown number of compounds. Injection volume was 5 μL. Dilution factor for Sample set 2 was 4.
Chromatography: HPLC separation was performed on an ExionLC™ System using a Phenomenex Synergi Hydro-RP column (20 × 2 mm, 2.5 µm). Mobile phase A was ammonium formate in water and mobile phase B was formic acid in methanol.
Figure 2. Typical Setting to for Compound Identification in Positive Ionization Mode.
Mass Spectrometry: Data was collected using both data dependent acquisition and SWATH® Acquisition methods using an X500R QTOF system, operating in positive ESI mode.
Data Analysis: Data was processed in SCIEX OS Software 1.0. In this study post acquisition data processing was performed in a targeted screening approach, in which samples were evaluated against a targeted list of compounds. The main confidence criteria for screening that were used were mass error, RT error, isotope ratio difference, and library score leveraging the flexible and easy to use matching and visualization features of SCIEX OS.
Simplified Data Processing
In this study, post-acquisition data processing was performed in a targeted screening approach, in which samples were evaluated against a targeted list of compounds using SCIEX OS software. The MS/MS library (SCIEX Forensics High Resolution MS/MS Spectral Library) was used for MS/MS library matching on both IDA MS/MS and SWATH acquisition data. As more targeted compounds are needed, they can be added to the library and used to re-interrogate the data at a later time without the need for re-injecting the samples.
Figure 2 show typical settings for positive compound identification. What this means is that any preliminary qualifying positive identification needs to satisfy the following criteria: 1) peak is detected, 2) mass error is better than 5 ppm, 3) RT error is better than 10%, and 4) library matching score is better than 30%.
LC Performance
Previously, a 2-minute LC method using a similar 20 x 2.0 mm dimension column for a similar fast screening method was demonstrated.2 Here, the method was adjusted for better retention of the very polar species by extending the LC runtime by half a minute and using a different column (Hydro-RP). Sufficient retention of those very polar species was important to allow diversion of the salt-containing eluates in the beginning of the gradient to waste. Better distribution of all the eluting analytes throughout the gradient (Figure 1) reduced the number of co-eluting analytes, minimizing ion suppression and matrix effects. Sufficient organic wash at the end of the gradient was needed along with ample aqueous equilibration to maintain RT reproducibility and elongate column life. Within the 2.5-min LC runtime, resolution of several groups of isomers was achieved, as highlighted in Figure 1.
Table 1: Compound List for Set 1. This table shows the list of compounds analyzed in this study and the cutoff concentration required.
Figure 3. Quantitation and Identification Results in the Same Interface for JWH 210 5-OH Metabolite in Urine. In Analytics, identification and quantitation results can be viewed in one flexible view. Library matching results are shown in the table and the quality of matches can be easily assessed with the ‘Traffic Light” system, the more green checks the higher the identification confidence. The actual scores for the library matches are shown as well. When a compound is selected in the table, the quantitation results are shown below, with XICs on the left and concentration curve results on the right.
Identification and Quantitation
In the Analytics portion of SCIEX OS, identification by MS/MS spectral library matching and quantitation can be performed and results easily visualized. The MS/MS spectrum provides a “fingerprint” of the structure of a compound and spectral matching provides a powerful piece of evidence for a confident identification. Ramped collision energy (20 to 50 V) is used when collecting MS/MS spectral during spectral library generation to generate fragment rich MS/MS spectra. In this study, for both IDA and SWATH acquisition, the MS/MS data was collected in the same way for the best match between the acquired data and the spectral library.
For quantitation, the TOF MS data from both IDA and SWATH acquisition was used. Use of the accurate mass TOF MS full scan data provides the advantage of a generic methodology. It is a non-targeted method that allows later data re-interrogation to serach for unanticipated drugs. This is particularly important in the scenario of designer drugs where new drugs emerge on a monthly basis. But full scan TOF MS approach, in a lot of cases, is not selective enough when analyzing biological samples where matrix interference is common. With SWATH acqusiition, the MS/MS data can also be used for quantitation which often provides higher specificity in complex matrices. This was not tested here but has been described in a previous technical note. 1
Post-acquisition processing involved performing simultaneous quantitation and targeted identification. For targeted identification, mass error, retention time error and library score were used for confirmation. Viewing results at either the sample or compound level, the processed can be quickly evaluated for both quantitation and screening results, as shown for JWH 210 5-OH metabolite in urine (Figure 3).
Urine Sample Set 1
The first sample set consisted of urine samples spiked with all the compounds in Compound List 1 (Table 1) at various concentrations. These samples spiked with these 85 analytes would be considered “challenged” samples because there would not be any real case sample containing all these analytes. But these samples will be valuable in the evaluation of these two acquisition methods for screening.
Because the urine was spiked with known compounds, the detection rates for both acquisition techniques could be compared and a true positive rate was computed (Table 2).
For data dependent acquisition strategy, the true positive rate ranged from 86% at the lower concentration to 100% at the higher concentration. This is due to a few factors, mainly that MS/MS was not triggered on the compound or the MS/MS was weak and yielded a poor MS/MS library matching result. This was understandable as the ultra-fast chromatography increased the number of co-eluting substances and therefore the chance of missed MS/MS acquisition.
Using SWATH acquisition, at an equivalent concentration level, the true positive rate was typically better than with DDA. This is because MS/MS is acquired on all precursors all the time, so nothing is missed. Library scores and therefore detection can sometimes be negatively affected by the complexity of MS/MS spectra with the SWATH acquisition approach but on aggregate, SWATH performed better in this challenged urine sample.
Figure 4. Extracted Chromatograms of Parent Ions of Selected Analytes in Urine. XICs were generated on a few select compounds using a 10 mDa extraction window around their accurate masses at 50% CO1 concentrations.
Table 2. True Positive Rate in Urine Sample Set 1 for IDA and SWATH Acquisition Strategies. For the fast chromatography, SWATH acquisition provided more comprehensive detection as expected, especially at lower concentration levels in matrix.
Screening and quantitation performance for all the 84 compounds was overall satisfactory with this ultra-fast LC method for diluted urine samples. The mass errors for all these analytes at all the tested concentrations were mostly within 2 ppm. The retention time consistency was excellent.
Figure 10 shows the extracted ion chromatograms for a few analytes with much lower cutoff concentrations such as 6-MAM, acetyl fentanyl, buprenorphine, fentanyl, norbuprenorphine and norfentanyl, with a 10 mDa extraction window around their accurate masses at 50% CO1 concentrations.
Urine Sample Set 2
Sample set 2 consisted of both urine samples spiked with known compounds and a set of urine samples with unknown compounds in them. The “challenged” standard mixtures were blank urine samples spiked with various concentrations of a different compound panel from Table 1.
A similar statistical analysis was performed to compare the positive rate in the Set 2 standard mixtures in urine from IDA and SWATH acquisition data (Table 3). Similar to what was observed in sample set 1, the positive rate was slightly better for SWATH acquisition data.
20 urine samples including samples with unknowns were used to further demonstrate the screening performance of this ultra-fast method. Sample 1 and 2 were quality control urine samples spiked with all the analytes at different levels. Sample 3 through 20 were true unknown samples. There was a total of 82 true positive identifications from Sample 3 to Sample 20.
A longer 6.5-minute method was also compared to the fast 2.5- minute method developed here and both IDA and SWATH acquisition were used (Table 4). With the 6.5-minute method, the true positive rates were both 96.3% for IDA and SWATH acquisition. True positive rates were lower (84.2%) for the IDA with the shorter 2.5-minute method due to the higher possibility of missing MS/MS scans. For the 2.5-minute SWATH acquisition, the true positive rate was significantly improved at 93.9% (Table 4). Quantitation was again performed with TOF MS information and the peak area results from the 6.5-minute and 2.5-minute methods agreed (data not shown).
Table 3. True Positive Rate in Urine Sample Set 2 for IDA and SWATH Acquisition. Again, SWATH acquisition provided more comprehensive detection at lower concentration levels in matrix for the fast chromatography,.
Table 4: Screening Performance for Unknown Samples. For the unknown samples, both IDA and SWATH acquisition were used in combination with the 6.5 min and the fast 2.5 min gradient methods. Compounds detected with confidence in each sample are listed in this table. In addition, the detections from the challenged samples in this sample set were used to compute true positive detection rates.
Table 4: Screening Performance for Unknown Samples - Continued.
Conclusions
In this technical note, a super-fast screening/quantitation method (under 3 minutes) using the SCIEX X500R QTOF system has been demonstrated for use in a forensic laboratory. Two data acquisition methods for screening were tested and compared, the data dependent IDA method and the data independent SWATH acquisition method.
Both acquisition strategies have been shown to be very robust for screening, even when coupled with the very fast chromatography. However when throughput is the priority, and a short LC method was used, the results from this study suggests the preferred data acquisition approach would be SWATH acquisition. The results from Urine Sample Set 2 clearly demonstrated the nearly equal screening performance between the 6.5-minute and 2.5-minute methods when SWATH acquisition was used. Also, SWATH acquisition yielded better true positive rate than IDA due to its complete coverage of MS/MS information. When a longer LC method was used, IDA and SWATH acquisition gave equally excellent performances.
References
- Using SWATH® Acquisition for Forensic Designer Drug Analysis with SCIEX X500R QTOF System and SCIEX OS Software. SCIEX Technical Note, RUO-MKT-02-4542-A
- Forensic Identification and Quantification Workflows Delivered on a Revolutionary Designed QTOF and SCIEX OS Software. SCIEX Technical Note, RUO-MKT-02-3786-A
 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge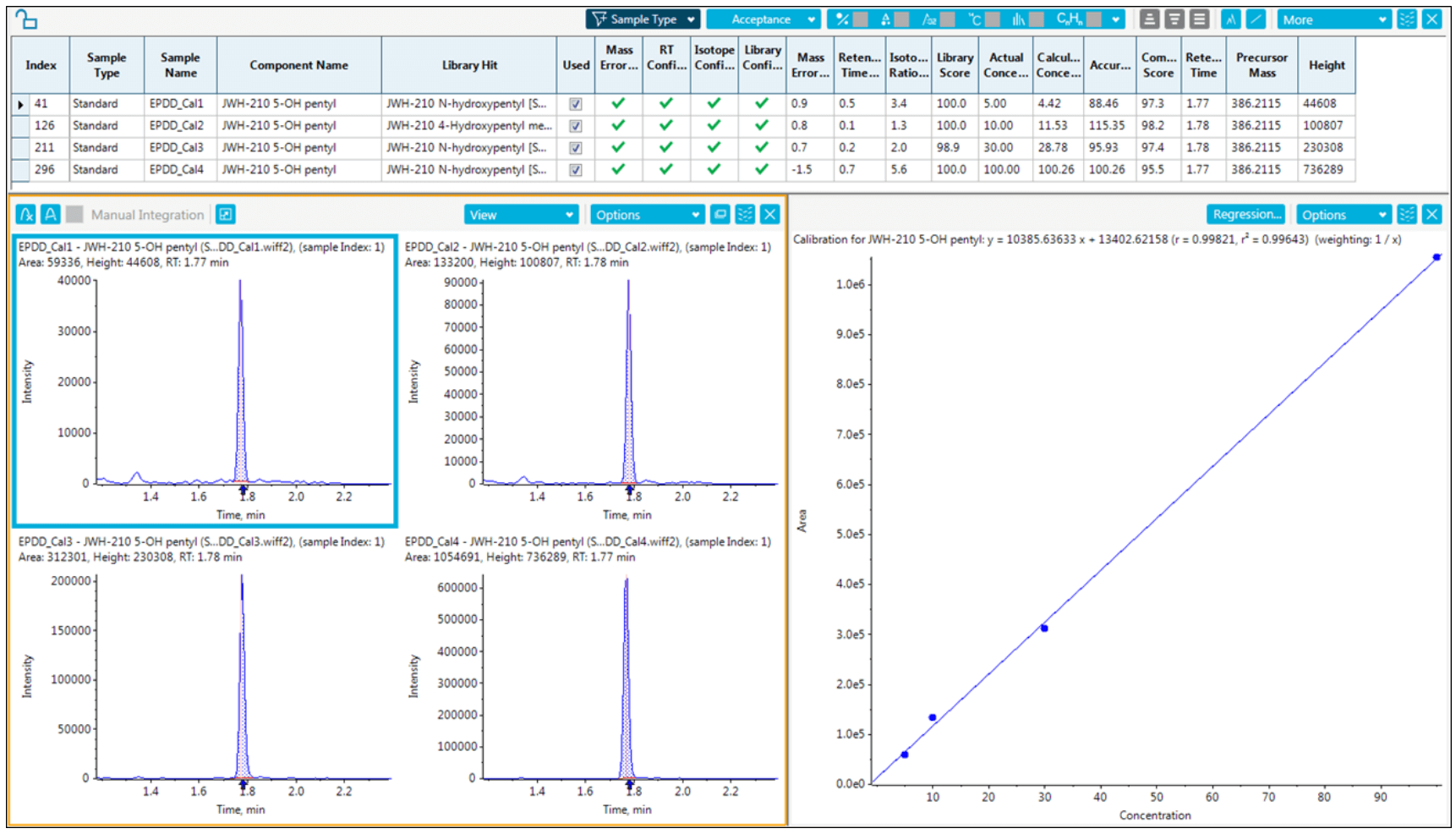 Click to enlarge
Click to enlarge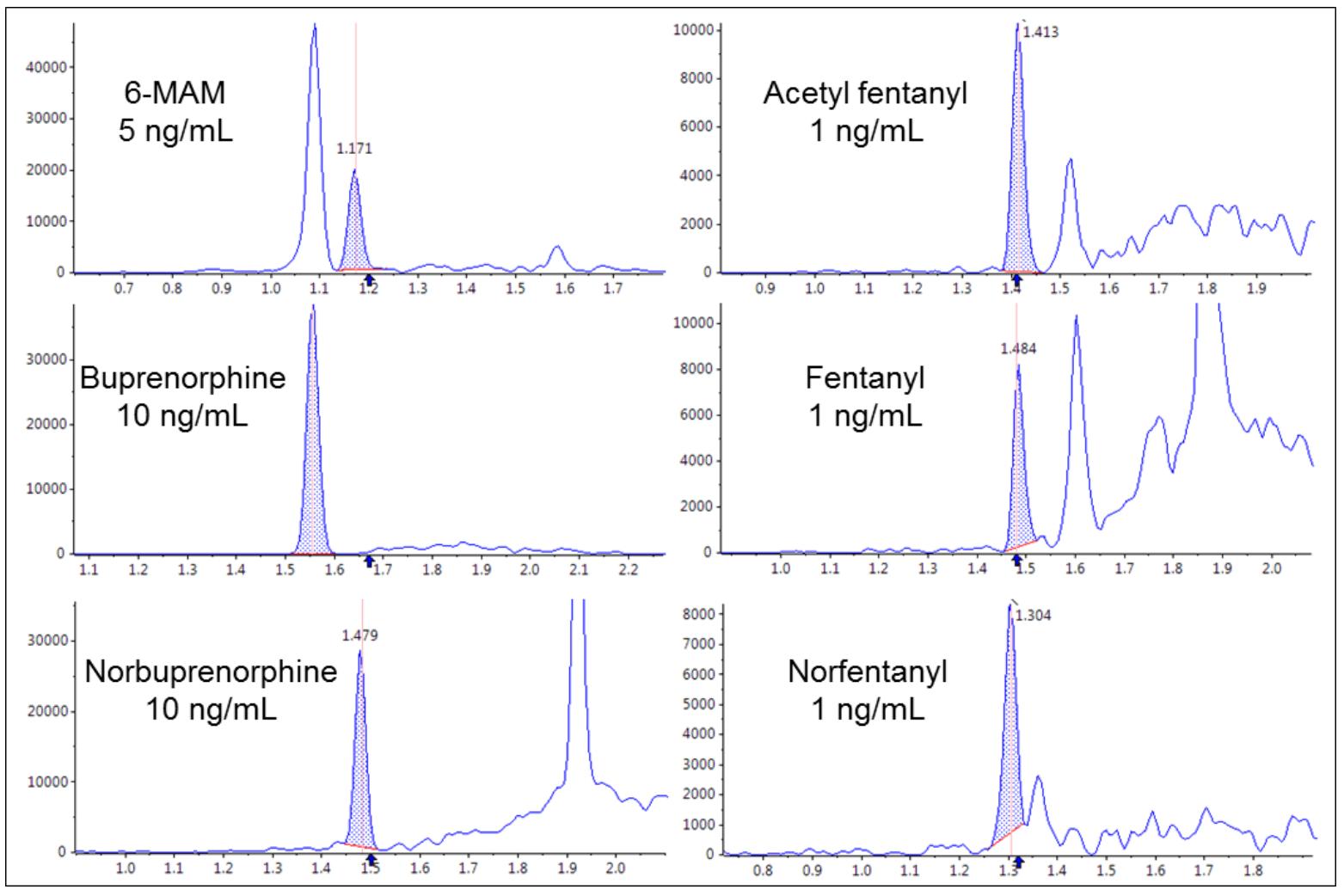 Click to enlarge
Click to enlarge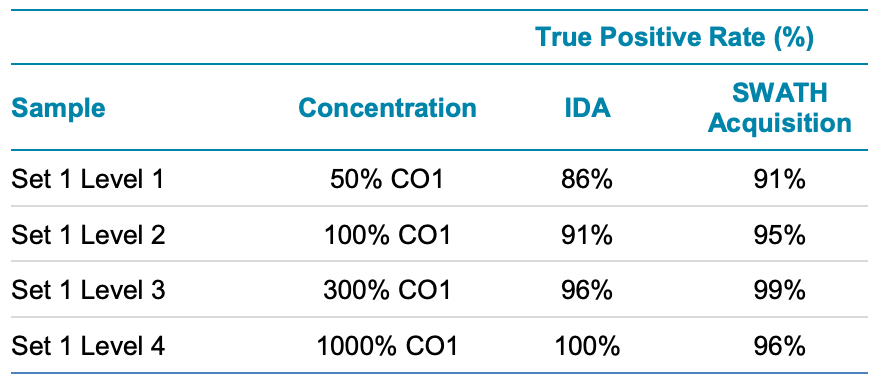 Click to enlarge
Click to enlarge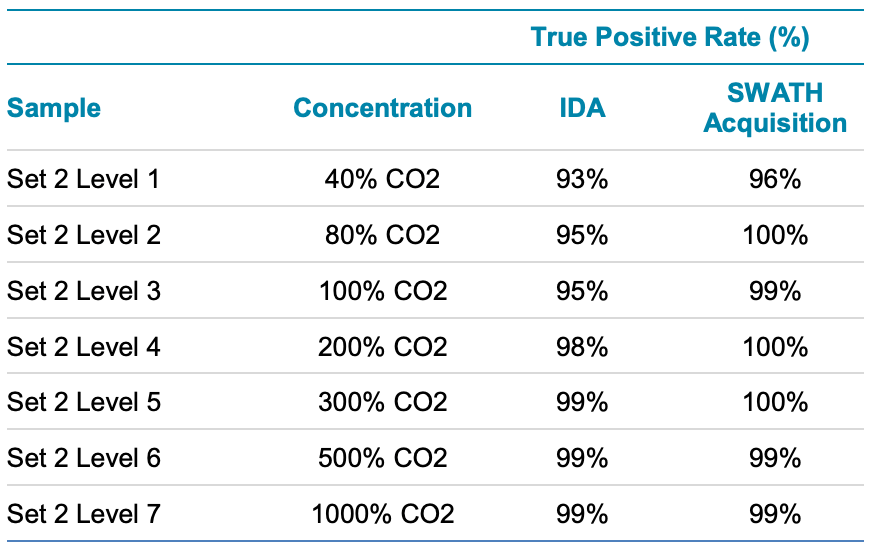 Click to enlarge
Click to enlarge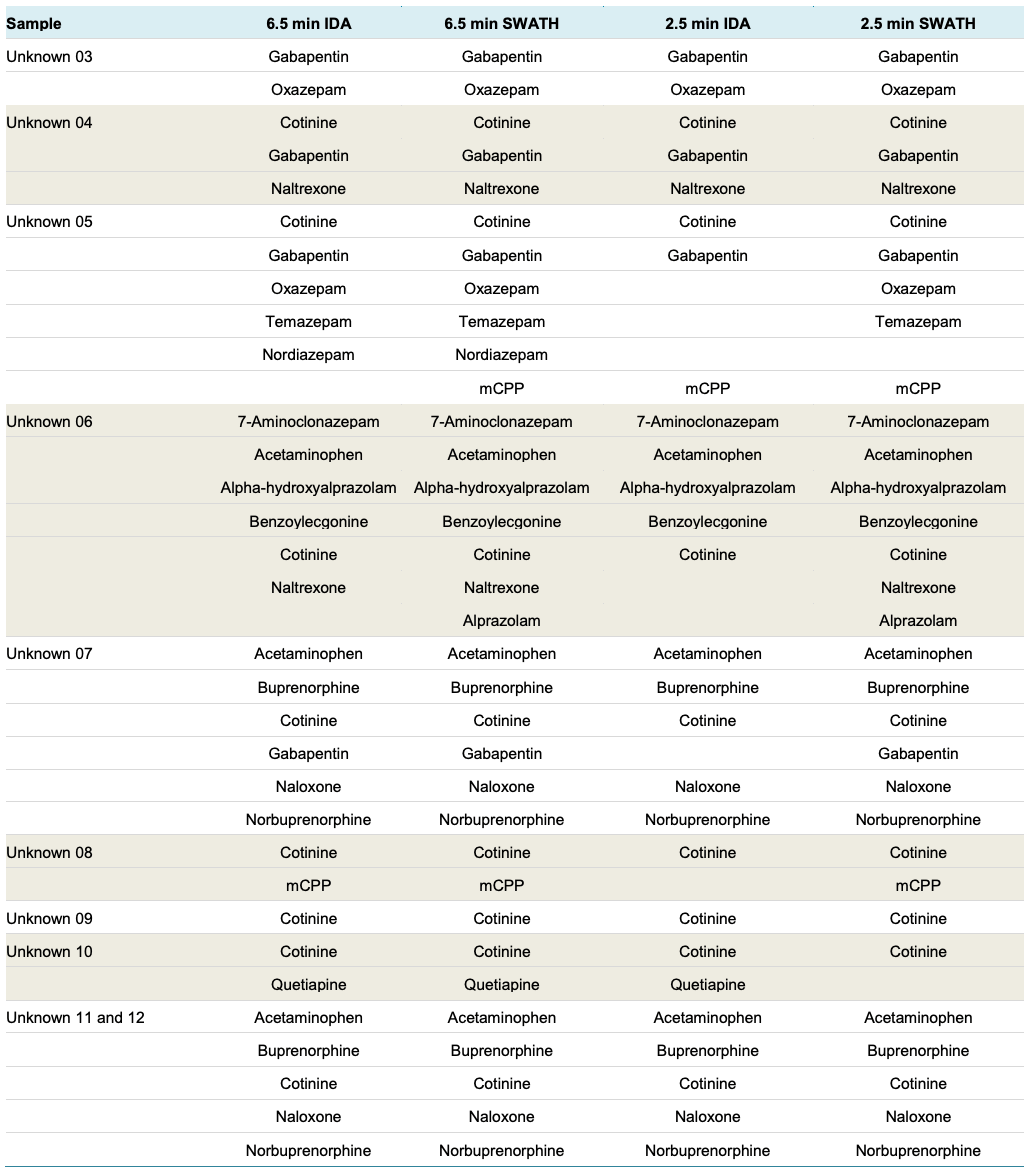 Click to enlarge
Click to enlarge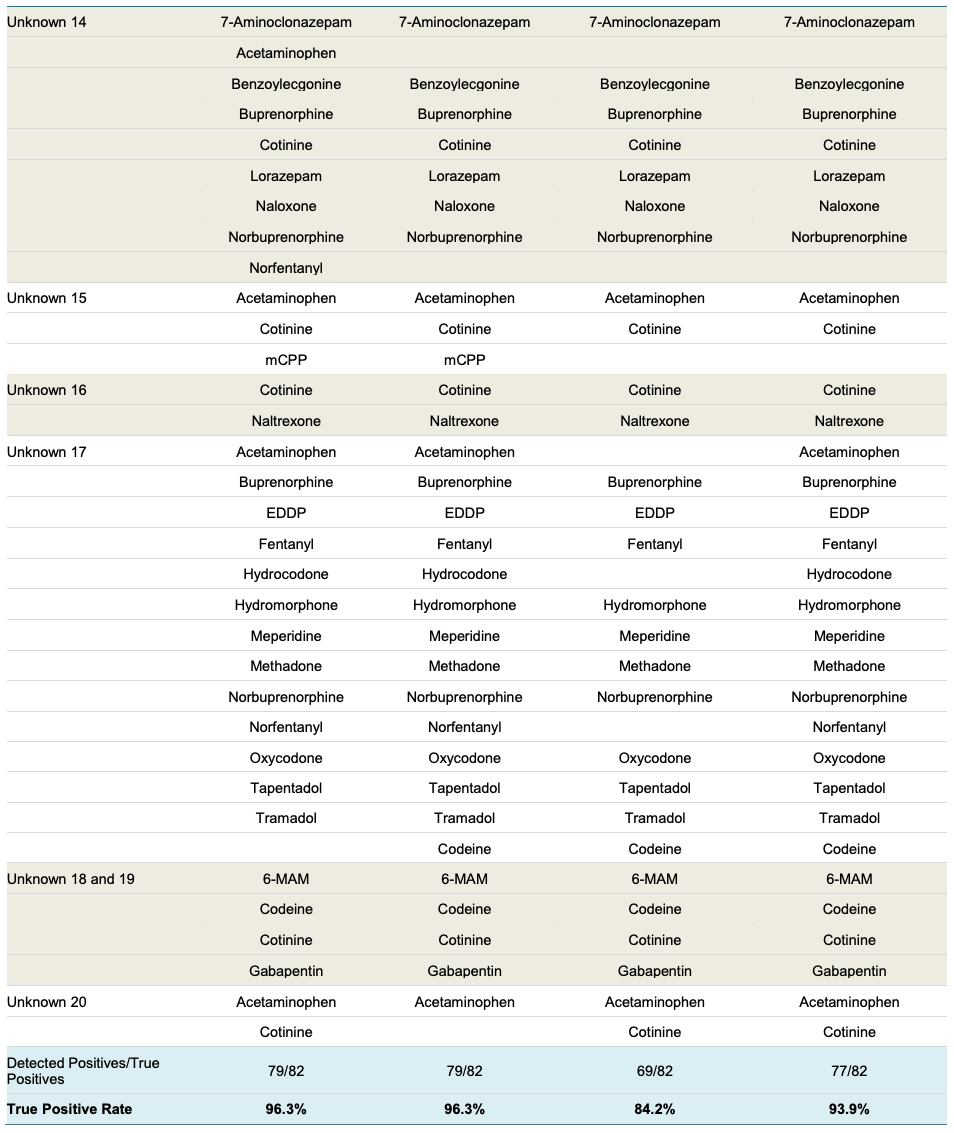 Click to enlarge
Click to enlarge