High system robustness enables analysis of large-scale sample sets
With the SCIEX QTRAP® 6500+ LC-MS/MS System
Introduction
Robustness is one of the key measures of successful and long-lasting LC-MS/MS analysis. This is easily achievable when utilizing the QTRAP 6500+ LC-MS/MS System with the IonDrive™ Turbo V Ion Source and Curtain Gas™ Interface. The simplicity of design in both the source and interface leading to highly stable and robust sample introduction has made high throughput targeted analysis routine.
Here, the robustness of the QTRAP 6500+ LC-MS/MS System was tested during the analysis of gut metabolites in plasma. This was a challenging study both due to the high number of samples to be analyzed and the complexity of the matrix and excipients used.

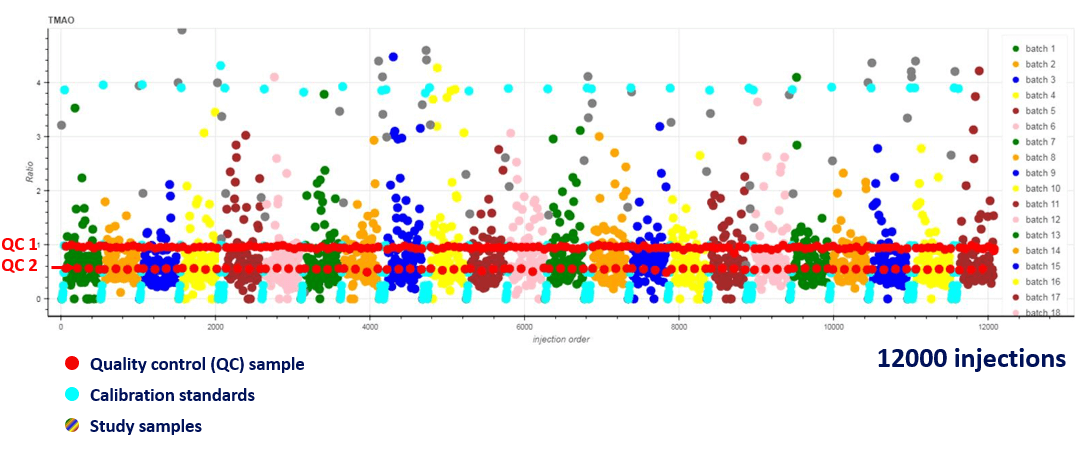
Figure 1. Robustness observed over 12000 concurrent injections of gut metabolites in a plasma matrix. Extremely high consistency in the quantification of L-carnitine and choline was observed over a vast number of injections, especially when focusing on the quality control samples shown in red and the calibration standards in blue. The study samples are a range of colors and show the numerous batches that were analyzed (23 batches in total).
Results
L-carnitine and choline are two of the gut metabolites which were studied as they have been found to increase the risk of cardiovascular disease in humans due to their conversion to trimethylamine N-oxide (TMAO) through various intermediaries; driving increased interest in high throughput LC-MS analysis.
A fast LC-MS/MS method was devised to analyze the various gut metabolites in a plasma matrix. The development of this was necessary due to the excipients associated with the matrix, meaning that retention of these components, namely phospholipids was needed to combat the ion suppression which was observed.
This then allowed for the gut metabolites, which will not be retained by the column (C18 stationary phase) to be analyzed more easily and without as much matrix interference, leading to an improved and more stable analysis when compared to the flow injection analysis which was first implemented.
An example of the system robustness over 12000 injections is outlined in Figure 1. Highlighting that even in a plasma matrix, the SCIEX QTRAP 6500+ System can provide consistency and throughout across thousands of injections without the need for any instrument cleaning or maintenance.
Conclusions
To summarize, the SCIEX QTRAP 6500+ LC-MS/MS System provides an extraordinary level of robustness across very large scale projects, even in the most challenging of matrices. This leads to higher instrument uptime and higher project throughput. This is in addition to the demonstrated high levels of sensitivity and specificity inherent to the SCIEX QTRAP 6500+ Systems.
 Click to enlarge
Click to enlarge