Abstract
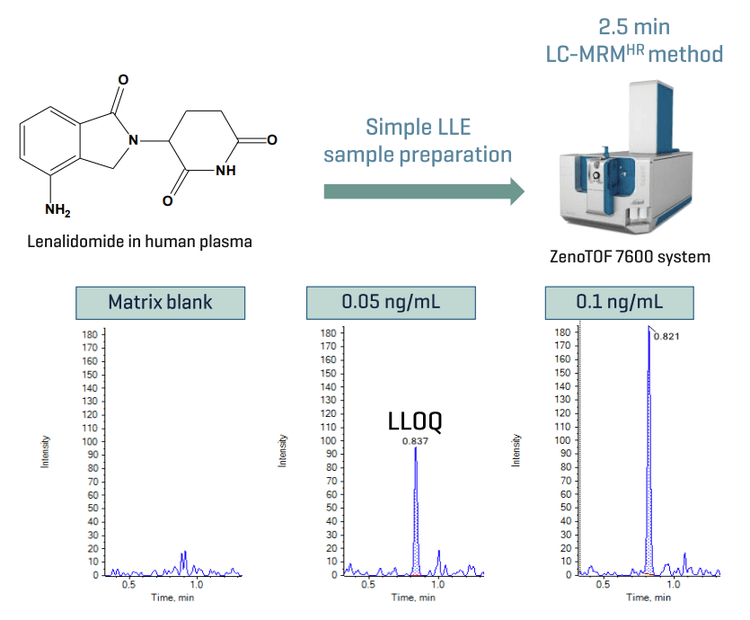
This technical note demonstrates a sensitive method for the quantitation of lenalidomide in human plasma using Zeno MRMHR on the ZenoTOF 7600 system. A lower limit of quantitation (LLOQ) of 0.05 ng/mL was achieved with simple sample preparation and a fast runtime of 2.5 min (Figure 1).
Lenalidomide was approved by the FDA in 2005 and is used to treat certain cancers (multiple myeloma, follicular lymphoma).1 It is an analog of thalidomide and is bound to cereblon. Lenalidomide can target numerous myeloma cells, thus showing greater efficacy against multiple myeloma.2 Lenalidomide is initially administered at a low concentration of 2.5 or 5 mg, adjusted based on the patient's renal function.3 Therefore, when assessing levels of lenalidomide in biological matrices for toxicokinetic and pharmacokinetic profiles, it is crucial to employ sensitive assays to help effectively evaluate the efficacy and the safety of the drug.
Key benefits for analysis of lenalidomide using the ZenoTOF 7600 system
- Sensitive quantitation of lenalidomide: Achieve 0.05 ng/mL LLOQ for the quantitation of lenalidomide in human plasma
- Low plasma consumption and simple sample preparation: Use a low sample volume of 100 µL of human plasma paired with a simple liquid-liquid extraction (LLE) methodology to quantify lenalidomide
- Fast analysis: Perform sensitive LC-MRMHR analysis of lenalidomide in human plasma in 2.5 min
- Effortlessly meet critical quantitative performance criteria: Achieve accurate quantitative performance with %CV <8 at all concentration levels across a linear dynamic range (LDR) of 3 orders of magnitude
- Streamlined data management: SCIEX OS software, a 21 CFR Part 11-compliant platform, simplifies data acquisition and processing
Introduction
Lenalidomide is a derivative of thalidomide used in treating multiple myeloma and has excellent efficacy against myelodysplastic syndrome.4 It works by reducing the synthesis of cytokines and growth factors, improving the immune system, or preventing the development of new blood vessels in tumors. Because of their selectivity towards tumors, lenalidomide has a high level of efficacy with minimal adverse effects.
Due to its high potency in treating malignancy, highly sensitive assays are necessary to ensure precise and accurate detection and quantitation when assessing pharmacokinetic and pharmacodynamic effects.
Methods
Standard preparation: 1 mg of lenalidomide stock was procured from Medchem Express and dissolved in methanol. The spiking solutions were prepared using 10:90 (v/v) acetonitrile: water, with 0.1% formic acid as diluent.
Sample preparation: Lenalidomide was spiked into 100 µL of human plasma at concentrations ranging from 0.05 to 50 ng/mL. To 100 µL of the plasma sample, 2.5 mL of ethyl acetate was added and vortexed for 10 min . The samples were centrifuged at 1204 rcf for 5 min. The supernatant was collected and dried under a nitrogen stream at 40°C. The dried samples were reconstituted in 300 µL of diluent.
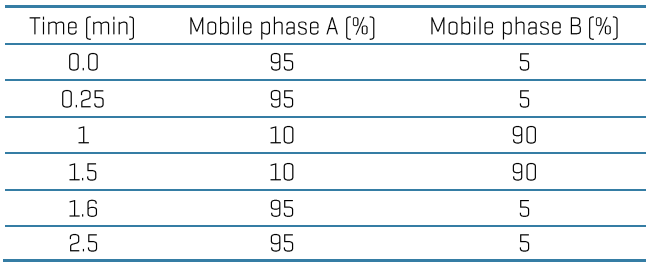
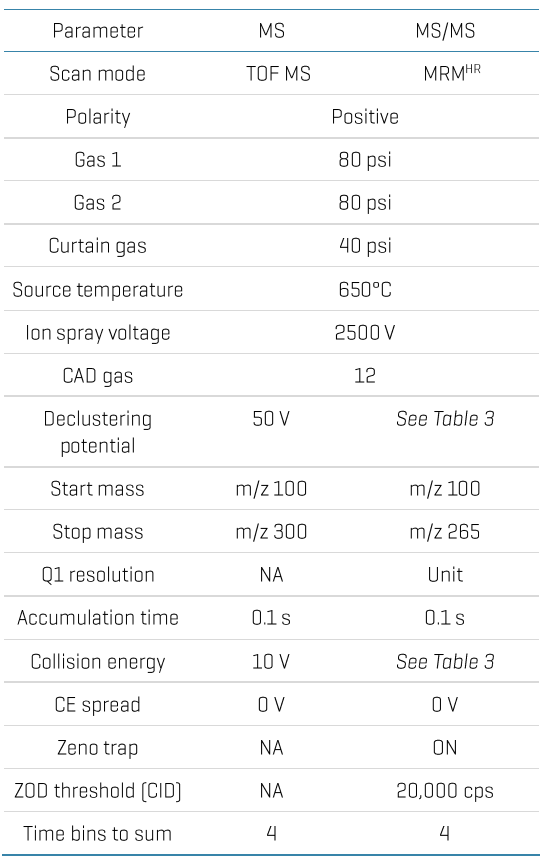
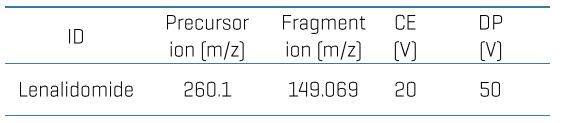
Chromatography: Analytical separation was performed on the ExionLC AE system using a Luna omega polar C18 column (50x2.1 mm, 5 µm) at a 0.7 mL/min flow rate. Mobile phase A was 0.1% (v/v) formic acid in water, and mobile phase B was 0.1% (v/v) formic acid in acetonitrile. The column temperature was set to 50°C. The gradient conditions used are summarized in Table 1. A 5 μL sample was used for LC-MS/MS analysis.
Quantitative performance on ZenoTOF 7600 system
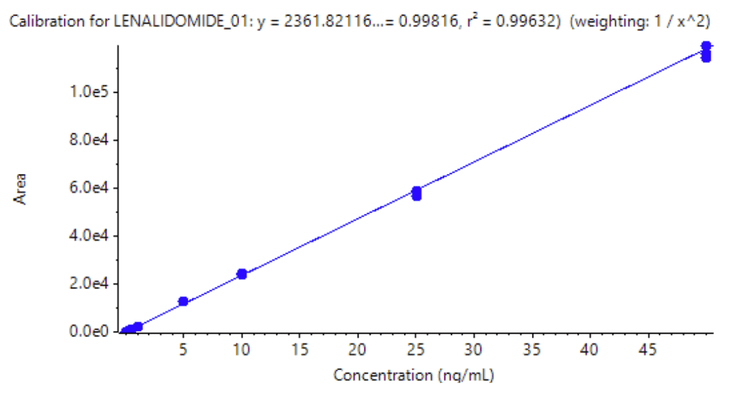
A triplicate injection was performed across concentrations ranging from 0.05 ng/mL to 50 ng/mL (Figure 2). An LDR of 3 orders of magnitude was achieved. A weighing factor of 1/x2 was used and a coefficient of determination (r2) of >0.996 was reached, showing excellent linearity across a wide calibration range.
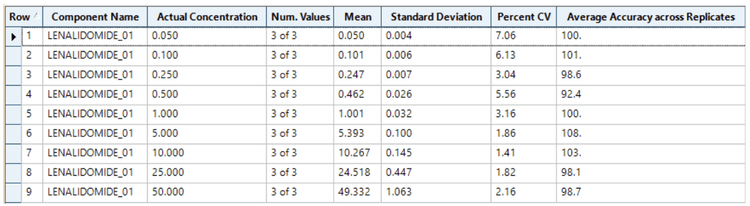
Analytical performance was evaluated based on the requirement that the accuracy of the calculated mean should be between 80% and 120% at the LLOQ and between 85% and 115% at higher concentrations. The %CV of the calculated mean of the concentration should be below 20% at the LLOQ and below 15% at all higher concentrations.5
This assay's accuracy was within ±8% of the nominal concentration, and the %CV was <8 for the quantitation of lenalidomide in human plasma (Figure 3). Calculated %accuracy and %CV values were within the acceptance criteria at each concentration level.
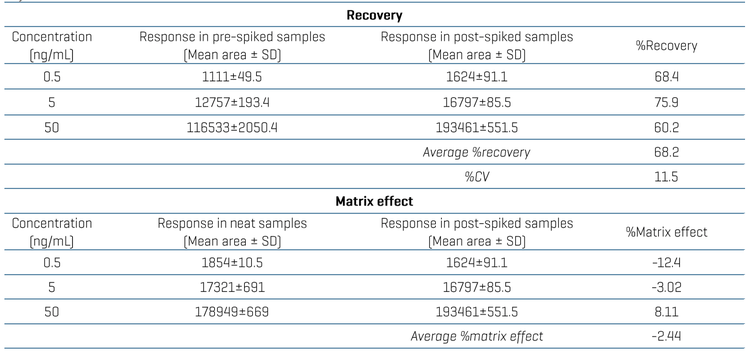
Recovery was evaluated for lenalidomide at 3 different concentrations (0.5, 5 and 50 ng/mL). A triplicate injection of the plasma-extracted sample was compared against the post-spiked samples. The average recovery was 68.2% with a %CV of 11.5, achieved using a simple LLE technique (Table 4).
The matrix effect was evaluated at 3 concentrations (0.5, 5 and 50 ng/mL). A triplicate injection of post-spiked samples was compared against the response in the neat solution (Table 4). The matrix effect was within the regulation limit of ±15% at 3 different concentrations.5 The average matrix effect was -2.44% as shown in Table 4.
Compliance-ready SCIEX OS software
Equivalent SCIEX OS software capabilities for regulated bioanalysis can be executed on the ZenoTOF 7600 system,
ensuring high fidelity when performing method transfers while retaining critical compliance features. SCIEX OS software is a closed system and requires records and signatures to be stored electronically, meeting the regulations outlined by 21 CFR Part 11. SCIEX OS software can open raw data files from any visible storage location within a closed network by using designated processing workstations.
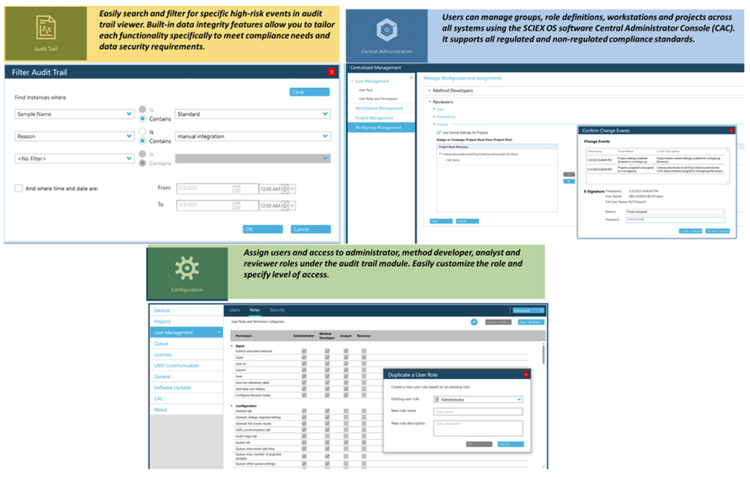
Figure 4 illustrates the features of SCIEX OS software used to monitor the audit trail, acquire and process data, and configure user access. The audit trail feature enables users to audit critical user actions and locks in data integrity. The Central Administrator Console (CAC) feature allows users to centralize acquisition and processing using a single platform to maximize efficiency for multi-instrument laboratories, independent of compliance standards.
The configuration module allows users to assign roles and access as the administrator, method developer, analyst, and reviewer.
Conclusions
- An LLOQ of 0.05 ng/mL was achieved for quantitation of lenalidomide in human plasma
- Low-level quantitation was achieved using 100 µL human plasma with a simple LLE technique using a fast LC method with a run time of 2.5 min
- Linearity was achieved at concentrations ranging from 0.05 ng/mL to 50 ng/mL, achieving an LDR of 3 orders of magnitude
- Good quantitative performance was demonstrated with accurate and highly reproducible (%CV <8) results on the ZenoTOF 7600 system
- A single platform for streamlined data acquisition, processing and management with SCIEX OS software was presented
- Retain data management and compliance-readiness (21 CFR Part 11) features using SCIEX OS software to support regulated bioanalysis on the ZenoTOF 7600 system
References
- FDA approves Lenalidomide (Revlimid), Feb 2017.
- Chen N, Kasserra C, Reyes J, Liu L, Lau H. Single-dose pharmacokinetics of lenalidomide in healthy volunteers: dose proportionality, food effect, and racial sensitivity. Cancer Chemother Pharmacol. 2012;70:717–725.
- Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309.
- Sheeba K. Thomas, Tiffany A. Richards MS, Donna M. Weber MD. Lenalidomide in multiple myeloma. Feb 2008, 20 (4), 717-735.
- Bioanalytical Method Validation, May 2018