Using the SCIEX Triple Quad 4500 system
Junmiao Chen, Dandan Si and Zhimin Long
SCIEX, China
Abstract
Nitrosamine impurities can be created during the drug production process and need to be monitored in drug APIs or drug products because of their potential harmful impact on human health. During the synthesis of rifamycin antibiotics, two nitrosamine impurities are known to be potentially created, MNP (1-methyl-4-nitropiperazine) and CPNP (1-cyclopentyl-4-nitropiperazine). A method has been developed here for the quantification of MNP and CPNP in rifampin and rifapentine drug APIs. Method provides the sensitivity, reproducibility and recovery rate required for genotoxic impurity detection.

Introduction
Nitrosamine impurities can be created during the drug production process, through drug synthesis or various degradation pathways. These impurities have been shown to impact health by causing damage of cellular DNA, potentially producing mutagenic and carcinogenic effects.1 Nitrosamine impurities can have strong toxicity and can cause damage to human genetic material at very low concentrations, hence the need for specific and highly sensitive detection in active pharmaceutical ingredients (API) and final drug products. However, detection of such low-level impurities in the presence of very high concentrations of drug can pose analytical challenges.
Rifampin and rifapentine are rifamycin antibiotics that are often used in combination with other antibiotics to treat a variety of infections, including divergent bacillus infections such as tuberculosis. According to the FDA, the starting raw material for the synthesis of rifampin, 1-amino-4-methylpiperazine, can produce 1-methyl-4-nitropiperazine (MNP) under certain conditions. Also, rifapentine raw materials may produce 1-cyclopentyl-4-nitropiperazine (CPNP) impurities during the synthesis process. Both MNP and CPNP are nitrosamine impurities and are classified as probable human carcinogens.2
Here, an LC-MS/MS method was developed and tested for the quantification of these 2 genotoxic impurities in rifampin and rifapentine (Figure 1).3
Figure 1. Detection of nitrosamine impurities in rifamycin antibiotics. Shown here is the detection of 1-methyl-4-nitropiperazine (MNP) in a commercial rifampicin API (top) and 1-cyclopentyl-4-nitropiperazine (CPNP) in a rifapentine API received from a collaborator (bottom), each with 3 replicate injections.
Key features of the SCIEX Triple Quad 4500 system for the analysis of MNP and CPNP
- Low detection limits were achieved with good quantitative linearity
- Good reproducibility and high recovery rates were observed
- The developed LC-MS/MS method meets the needs of genotoxic impurity detection.
Methods
Sample preparation: Stock solutions of MNP and CPNP were made across a range of concentrations in methanol (0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100 and 200 ng/mL).
Rifampin API: A 100 mg sample of rifampin API was combined with 10 mL of pure methanol. The solution was vortexed well to mix, then shaken for 2 min and centrifuged at high speed for 10 min. The supernatant was removed for analysis.
Rifapentine API: A 100 mg sample of rifapentine API was combined with 10 mL of pure methanol. The solution was vortexed well to mix, then shaken for 2 min and centrifuged at high speed for 10 min. The supernatant was removed for analysis
Chromatography: Samples were separated using an ExionLC AD system with an Ace UltraCore Super PhenylHexyl column (2.5 µm, 50 x 4.6 mm). The column temperature was held at 35°C and the injection volume was 5 µL. The gradient conditions used are outlined in Table 1.
Mass spectrometry: The analysis was performed using the SCIEX Triple Quad 4500 system operating in positive ion mode. MRM analysis was used for compound detection and the parameters used are outlined in Table 2. Source conditions were ISV 4500 V, GS1 65, GS2 45, CUR 30 and TEM 450 °C.
Data processing: Data analysis was performed using the Analytics module in SCIEX OS software.
Table 1. Chromatographic gradient.
Table 2. MRM parameters for MNP and CPNP. Two MRM transitions were monitored for each compound.
Sensitivity and linearity
First, standard curves of the MNP and CPNP impurities were generated in methanol and analyzed across the concentration range of 0.1–200 ng/mL. Good linearity was observed for both compounds, with R greater than 0.998 for each (Figure 2). Both analytes were easily detected at a concentration of 0.1 ng/mL (Figure 3). Six replicate injections were performed at this concentration level and showed peak area reproducibility of 3.08% for MNP and 1.45% for CPNP.
Figure 2. Calibration curves for the nitrosamine impurities. Good linearity was observed across the concentration range analyzed (0.1–200 ng/mL).
Figure 3. Reproducibility of quantification of nitrosamine impurities at 0.1 ng/mL. Good peaks were observed at the lowest concentration analyzed (0.1 ng/mL). Six replicate injections were performed at 0.1 ng/mL and showed a peak area reproducibility of 3.08% for MNP and 1.45% for CPNP.
Recovery rate and reproducibility
Next, the compounds were spiked into the APIs at fixed concentrations to measure the recoveries and quantification reproducibility in drug matrix. MRM peaks for the 2 impurities in commercial rifampin and rifapentine APIs are shown in Figure 1. The MNP content in the un-spiked rifampicin injection was ~4.5 ng/mL and the CPNP content in the un-spiked rifapentine API was ~50 ng/mL. Therefore, MNP was spiked at 5 ng/mL and CPNP at 50 ng/mL. The recovery rate of the 5 ng/mL MNP standard solution added to the rifampicin injection was 91% and the precision of the 3 samples gave a %RSD=1.93%. The recovery rate of the 50 ng/mL CPNP standard solution added to the rifapentine API was 90% and the precision of the 3 samples gave a %RSD=1.44%.
Conclusions
In this technical note, an LC-MS/MS method was established to quantify MNP and CPNP impurities in rifampin and rifapentine APIs using the SCIEX Triple Quad 4500 system. The method provided good linearity for both impurities. The standard recovery rate was verified to be greater than 90%. The peak area %RSD was <5% across all the various experiments, indicating very good reproducibility. These results show that the sensitivity, reproducibility, and recovery rate of the method meet typical regulatory requirements for the detection of the 2 impurities.
References
- Control of nitrosamine impurities in human drugs – guidance for industry – FDA – February 2021
- FDA Updates and Press Announcements on Nitrosamines in Rifampin and Rifapentine
- Liquid Chromatography-High Resolution Mass Spectrometry (LC-ESI-HRMS) Method for the Determination of MNP in Rifampin and CPNP in Rifapentine Drug Substance and Drug Product. Sept 2020, U.S. FOOD & DRUG Administration.
 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge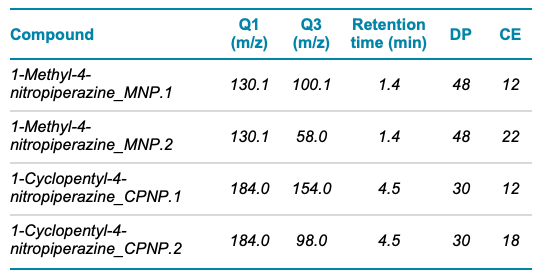 Click to enlarge
Click to enlarge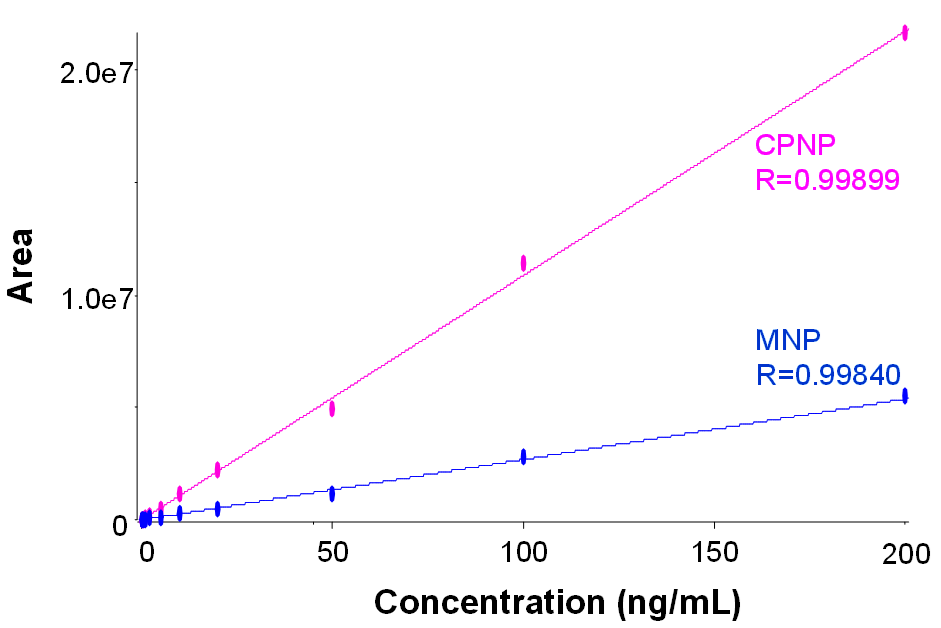 Click to enlarge
Click to enlarge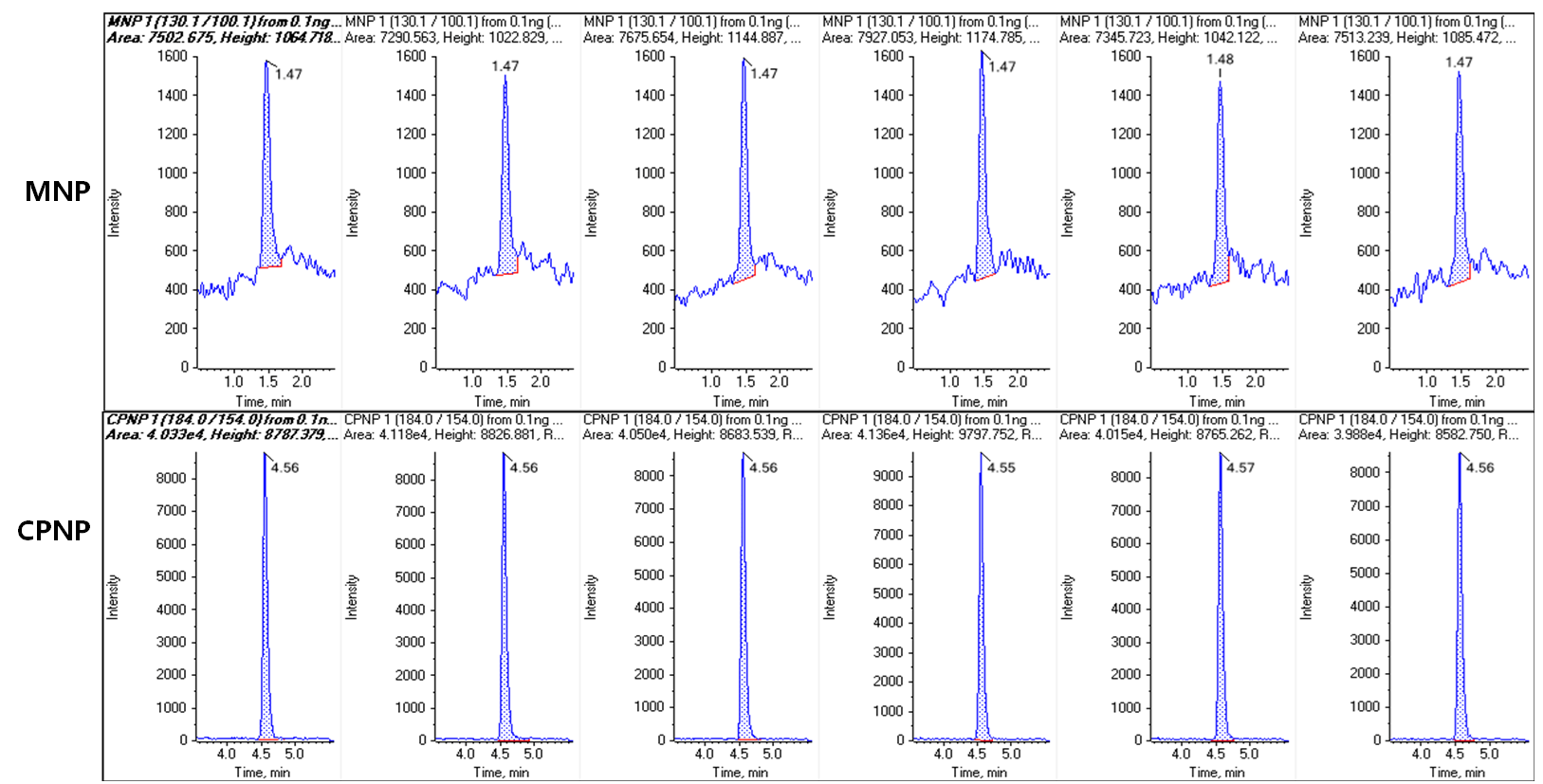 Click to enlarge
Click to enlarge