Abstract
Insulin-like growth factor(IGF)-1 and 2 are ~7 kDa protein hormones that are monitored in the study of human growth disorders. In biological matrices, approximately 99% of IGF-1 in blood is bound to six IGF-1 binding proteins (IGFBPs), while IGF-2 binds and interacts with IGF-1 receptor, IGF-2 receptor, IGFBP3 and transferrin. Dissociating IGFs from IGFBPs or receptors and preventing the reformation of these complexes are crucial for quantitative analysis of IGF-1 and 2.
Introduction
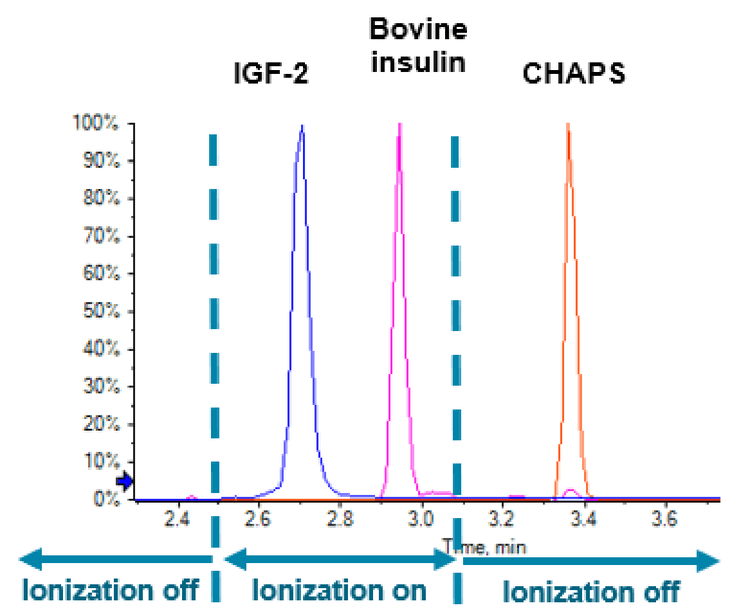
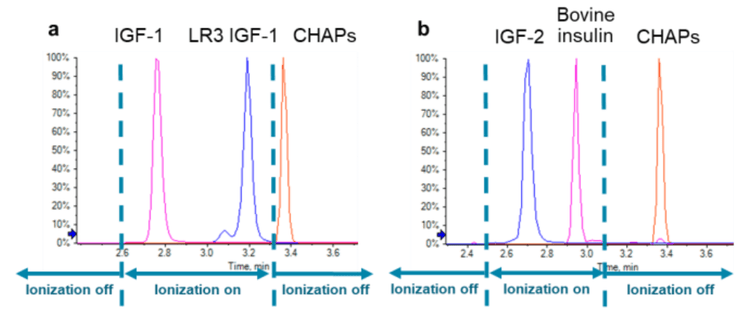
While traditional sample preparation methods using acidic alcohol or sodium dodecyl sulphate (SDS) meet significant challenges in obtaining optimal analyte recovery and sample cleanness, herein an MRM LC-MS/MS method coupled with mass spectrometry friendly sample preparation protocol utilizing CHAPS (3-[(3-cholamidopropyl) dimethylammonio]-1- propanesulfonate) is reported. The majority of CHAPS was removed from the samples during protein precipitation and SPE cleanup. 1 The remaining trace amount of CHAPS were base line separated from the analyte with the optimized HPLC condition and prevented from getting into the MS system by applying the scheduled ionization feature in Analyst® software 1.7 (Figure 1). With this method, IGF-1 and 2 were solidly quantified at 2 ng/mL level in serum samples by utilizing the QTRAP 6500+ LC-MS/MS system.
Key Features of the MRM LC-MS/MS workflow for intact IGF-1 and 2 quantitation
-
SCIEXQTRAP 6500+ and Triple Quad 6500+ mass spectrometer offers:
- Superior quantitation with high sensitivity, speed, reproducibility and robustness for biological analytes in the most challenging matrices
- Over five orders of linear dynamic range for enhanced breadth of applications
-
Analyst software 1.7 offers scheduled ionization feature to reduce the mass spectrometer downtime by decreasing the risk of contamination.
Methods
Sample Preparation: IGF-1 and 2 (Cell Sciences, Inc.) were serial diluted with 30% methanol, 60% water and 10% acetic acid. Long acting human IGF-1 analog, human LR3 IGF-1 (Cell Sciences, Inc.), was used as internal standard for IGF-1. Bovine insulin was used as internal standard for IGF-2. IGF standards and internal standards were spiked into 100 µL of mouse serum to prepare calibration curve samples. Samples were protein precipitated and loaded on Phenomenex® Microelution plate Strata-X-A 33 µm polymeric strong anion 96-well plate (2 mg/well) for SPE cleanup. The detailed sample preparation procedure is demonstrated in Figure 2.
Sample Preparation Optimization
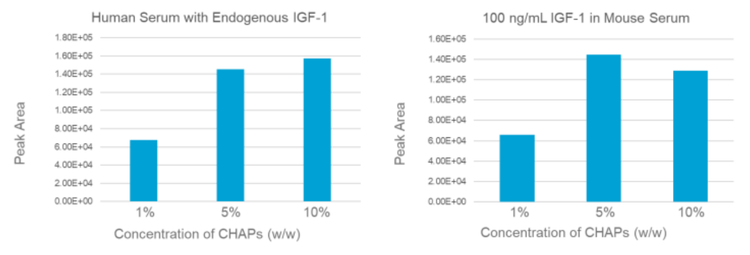
The analyte recovery of IGFs sample preparation highly depends on the dissociation efficiency between IGFs and their binding proteins and/or receptors. To optimize the dissociation process, various concentrations of CHAPs (1%, 5% and 10%) were evaluated for both human serum (Innovative Research, Inc.) and mouse serum (BioIVT) sample. The XIC peak areas of IGF-1 were used to evaluate the recovery rate. As shown in Figure 3, 5% and 10% CHAPS conditions provided the similar recovery which was ~2 folds higher than the 1% CHAPs condition. Therefore, 5% of CHAPs was used in the following work.
Scheduled Ionization to Minimize Instrument Contamination
As CHAPS is a zwitterionic detergent and can induce instrument contamination, additional method optimization practices were applied to minimize the amount of CHAPs that could enter the mass spectrometer. Firstly, HPLC conditions were well optimized to provide baseline separation between CHAPS and all analytes/internal standards (Figure 4). Secondly, the scheduled ionization feature in Analyst software 1.7 was turned on to apply ion spray voltage (ISV) only during the elution of analytes and internal standards. At the retention time of CHAPS, the ISV was set to zero (Figure 4), therefore no electrospray was form to allow CHAPs getting into the mass analyzer.
Quantitation Results
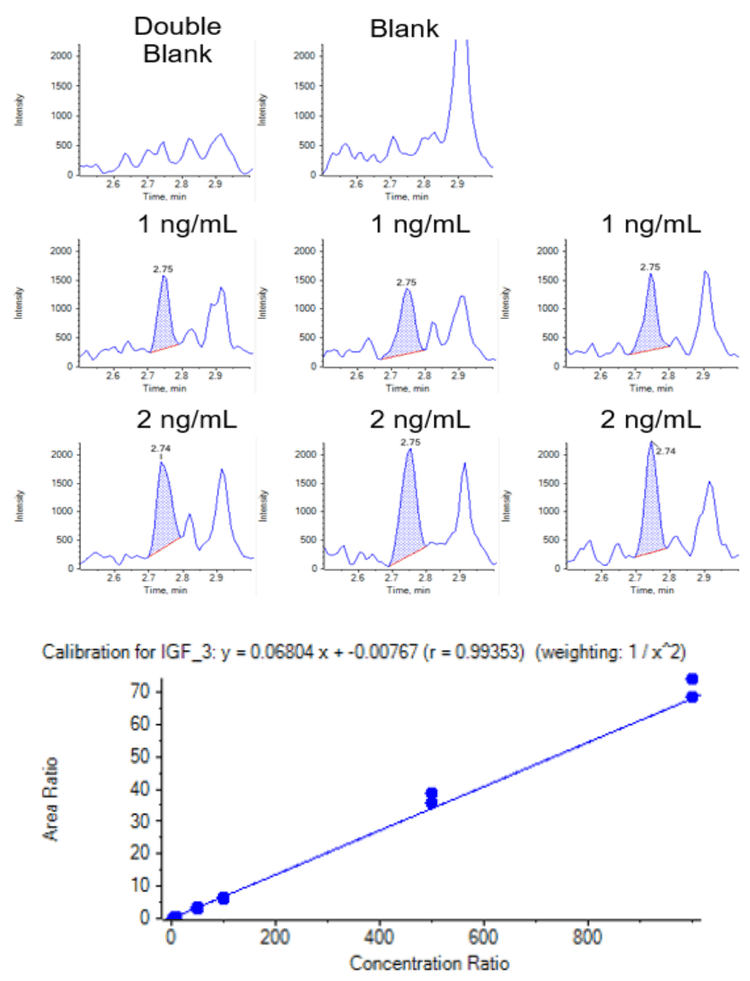
IGF-1 and 2 present endogenously in human serum, therefore mouse serum was used as a surrogate matrix to determine the linear dynamic range (LDR), limit of detection and quantitation (LOD and LOQ). The LDRs of IGF-1 and 2 were determined as 2-1000 ng/mL, with LOQ as 2 ng/mL and LOD as 1 ng/mL (Figure 5 and 6, Table 2 and 3). The pooled normal human serum was analyzed as an unknown sample and the concentrations of endogenous IGF-1 and 2 in this batch/lot of human serum were quantified as 119.4 and 397.6 ng/mL.
Conclusion
An MRM LC-MS/MS workflow for intact IGF-1 and 2 quantitation in serum samples was presented. Using the QTRAP 6500+ LCMS/MS system, IGF-1 and 2 were quantified at 2 ng/mL level with high reproducibility. Meanwhile, the Scheduled Ionization feature in Analyst software 1.7 efficiently prevented instrument contamination from sample preparation detergent (CHAPS). This workflow offers simplicity, speed and robustness, aligning up with the need in large molecule bioanalysis.
References
- Luo J, Guo L, Jin W, Xiong L, Quantitation analysis of human insulin-like growth factor 1 (IGF1) in serum by using SPE-LC-MS/MS workflow, SCIEX Technical Note, 2019, RUO-MKT-02-8964-A.




