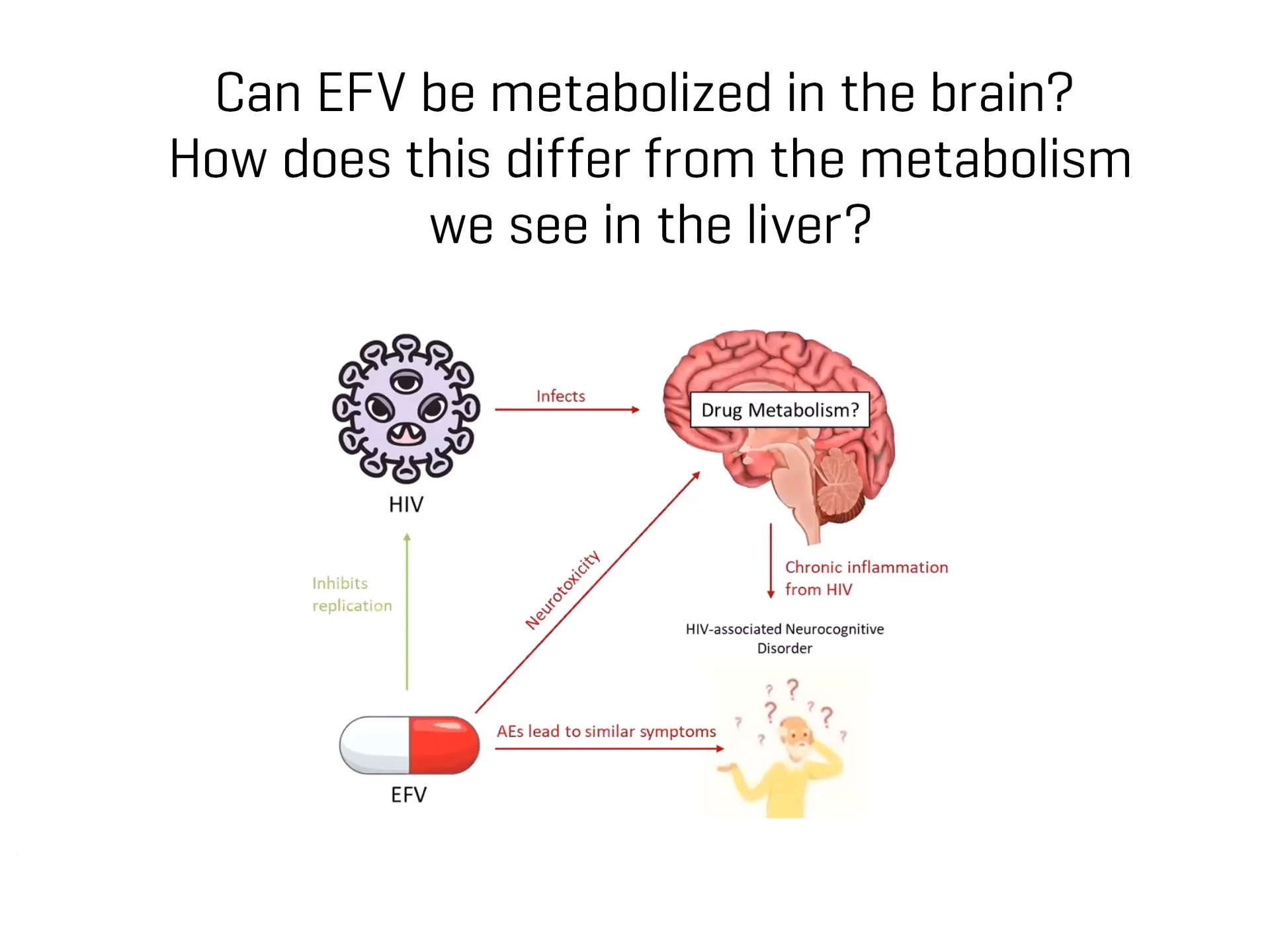
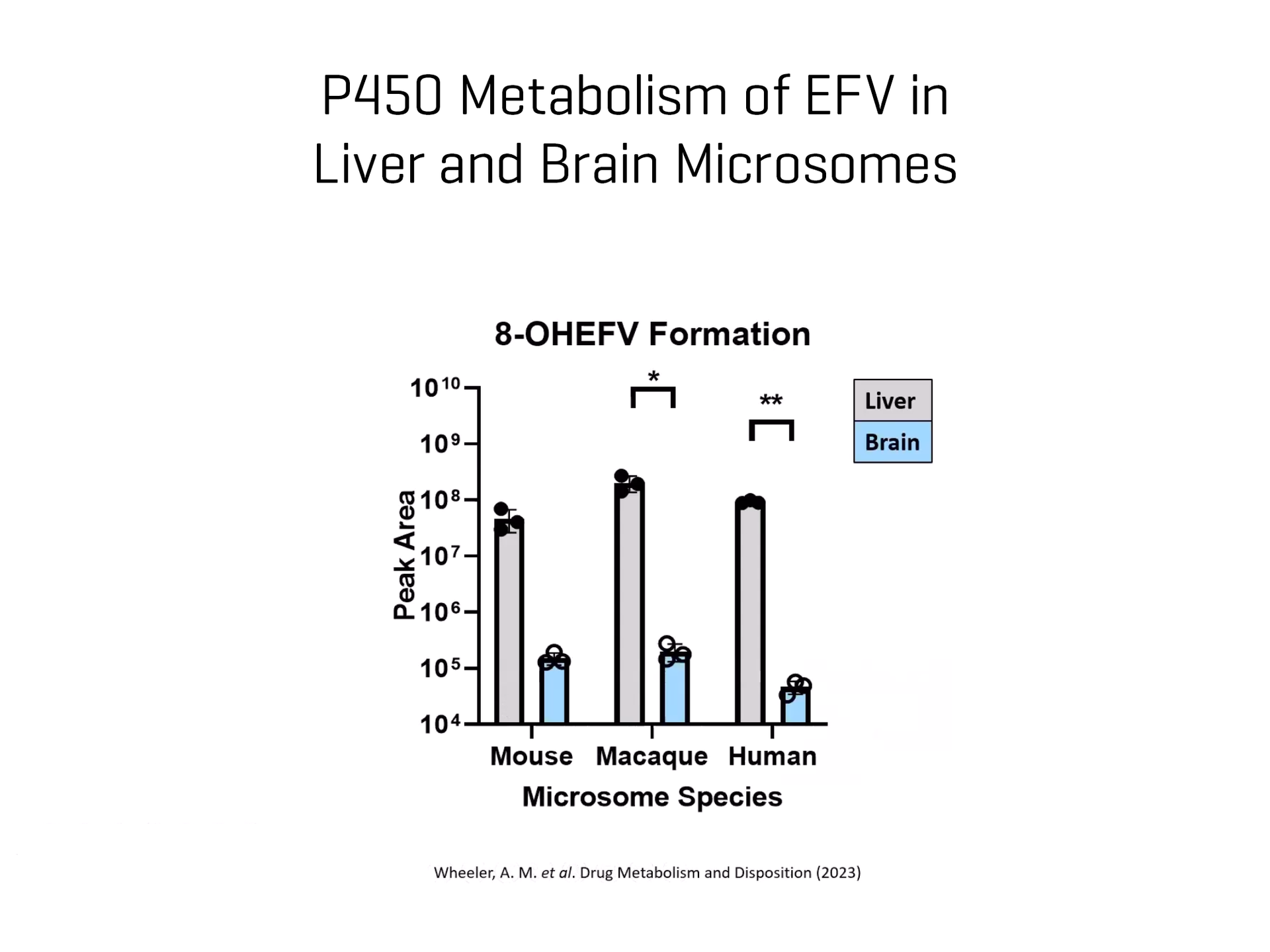
Drug biotransformation in the brain has often been overlooked in research. Historically, it was perceived that minimal drug metabolism occurred in the brain due to low concentrations of drug metabolizing enzymes and the blood-brain barrier acting as a physical roadblock for drugs and xenobiotics. However, the brain is not a homogenous organ. Recent studies have highlighted that drug-metabolizing enzymes are disproportionately concentrated throughout the different brain regions, with enzymes expressed at levels as high as, or higher than those in the liver in some regions.
The study of drug metabolism in the brain is crucial for advancing our understanding of pharmacokinetics (PK) and the effects of central nervous system (CNS) acting drugs. Valuable insights into drug exposure at the target site and its subsequent pharmacological and toxicological consequences can be inferred by understanding the presence, distribution and function of these drug metabolizing enzymes. This knowledge is critical for the development of effective and safe treatments, not only for neurological and psychiatric disorders, but also for other conditions requiring drugs that metabolize in the brain, such as HIV with antiretroviral medications.