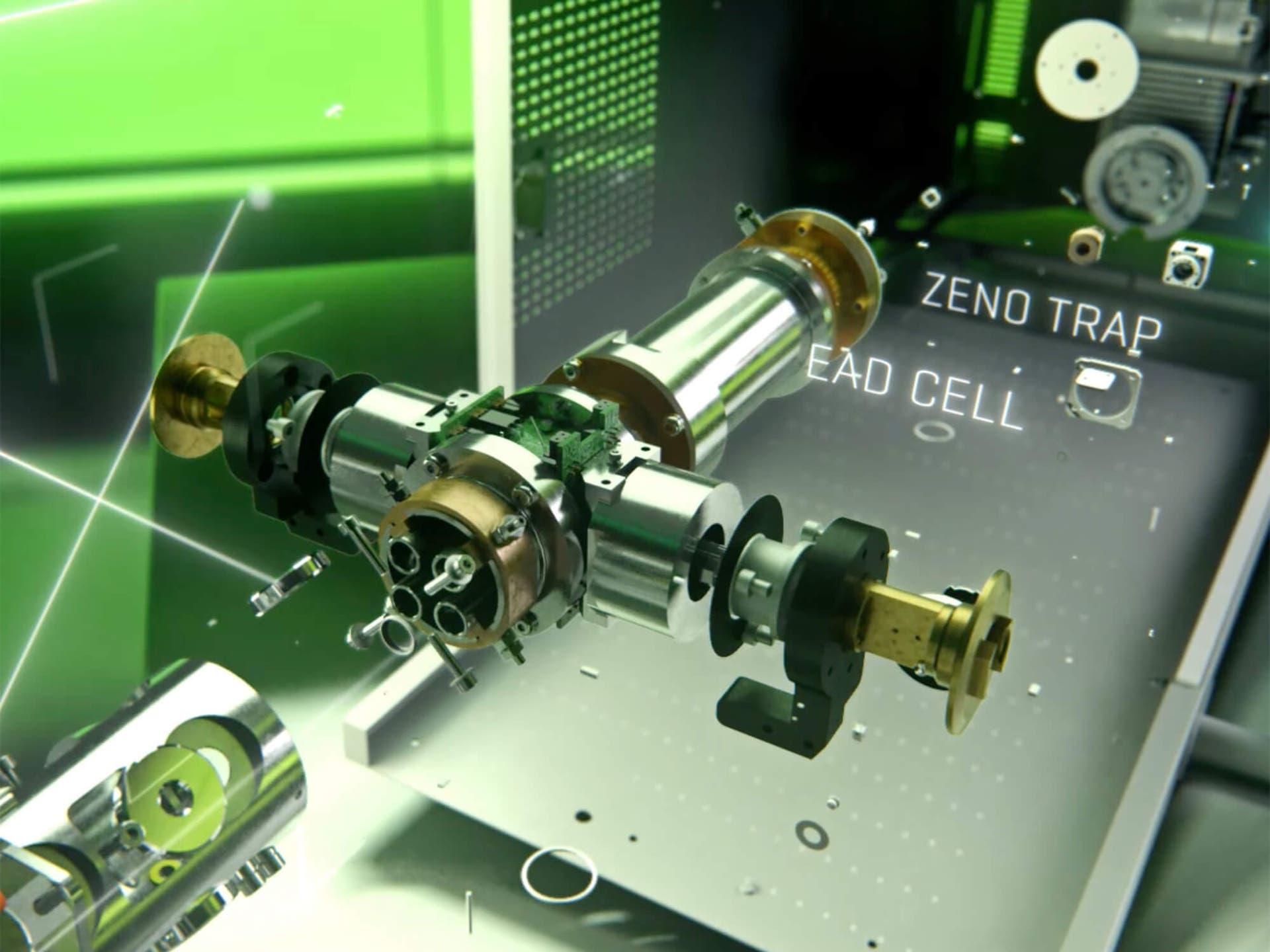
SCP is a technique used to study the proteins within individual cells. Proteins are important molecules that carry out many functions in our bodies. By studying them at the single cell level, scientists can gain a better understanding of how cells behave and communicate with each other. SCP allows scientists to identify and quantify the proteins present in a single cell, which can provide insights into how cells respond to changes in their environment, such as exposure to drugs or disease. This technique is particularly useful in the field of personalized medicine, as it can help identify the best treatments for individual patients based on their unique cellular responses.
Extraordinary science with Ben Orsburn
At the Johns Hopkins University School of Medicine, Ben Orsburn's research in single cell proteomics (SCP) using the ZenoTOF 7600 system is advancing personalized medicine by enhancing our understanding of cellular responses to drug treatments

Advancing personalized medicine with single cell proteomics (SCP)
Democratizing single cell proteomics with microflow time-of-flight mass spectrometry
SCP offers powerful new insights into basic biology and in evaluating the heterogeneity in the response of individual cells to drug treatments. This emerging field has been a boon for instrument vendors with multiple new and expensive instruments. New cell sorting technologies and sample handling robotics have increased both the reproducibility and efficiency of single cell sample preparations. In addition, ultra-low flow chromatography allows increases in the signal-to-noise ratio of both unlabeled and multiplexed samples. Although undoubtedly powerful, both approaches add new financial and technical barriers especially for those entering this emerging field.
In this study, Ben Orsburn describes an alternative approach in which single cells are prepared by low-volume 384-well suspension traps (S-Traps) using a simple standardized protocol that works for any cell sorting instrument or core service. He further explores the simplification of SCP by using a robust microflow chromatography system enabled by a new QTOF instrument with inline z-trap ion accumulation and an intrascan linear dynamic range of nearly 4 orders of magnitude. In addition, through direct links to cloud-based tools for peptide identification and interpretation, hundreds or thousands of single cells can be analyzed in minimal time without the need for dedicated bioinformatics support. To demonstrate the power of this approach, he analyzed single human pancreatic cancer cells treated with a newly described KRAS G12D inhibitor. The cell cycle status of each individual cell was easily identified by proteomic markers, which allowed the drug response to be evaluated independent of large proteomic alterations. Although other methods might yield deeper coverage of individual cells, the presented approach provides a workflow that is significantly more accessible to the wider scientific community than other leading approaches.
Key takeaways
- The promise of single cell proteomics in pharmacology
- The importance of intrascan linear dynamic range in SCP studies
- The implementation of multiplexed single cell proteomics

Watch Ben Orsburn's extraordinary science
About the presenter

Benjamin C. Orsburn, PhD
Instructor of Pharmacology and Molecular Sciences
Johns Hopkins University School of Medicine
Ben Orsburn received his PhD from Virginia Tech for using mass spectrometry to study the cell walls of bacterial pathogens. He completed postdoctoral fellowships at Johns Hopkins University and the National Cancer Institute, where he expanded his skills toward the transcriptomics and proteomics of cancer. He is the founder of News in Proteomics Research, which is the most frequently visited mass spectrometry and proteomics blog on the internet. He is dedicated to breaking down today’s most relevant advances in the field in the most approachable format possible. He is a contributing author to more than 40 scientific articles and editorials, in addition to 3 books on mass spectrometry. In 2018, he founded LCMSMethods.org, a site dedicated to improving proteomics and mass spectrometry through the release of instrument methods curated by subject matter experts. For this work, he was awarded a position in the Workflow Interest Network of the Association of Biomedical Research Facilities. His research has been featured in Science Daily, GenomeWeb and other media outlets. In March 2020, his work on accelerating the development of COVID diagnostic assays received mainstream media attention. Ben is now a faculty member at Johns Hopkins in the Department of Pharmacology and Molecular Sciences. His research is focused on the application of proteomics and single-cell proteomics toward a more comprehensive understanding of drug activity and toxicity.