Fast glycan labeling and analysis: high-resolution separation, quantification, and identification in minutes
Andras Guttman,1,2 Marton Szigeti,1 Anna Lou,2 Mervin Gutierrez,2
1SCIEX, Brea, CA 92822
2 Horváth Csaba Laboratory of Bioseparation Sciences, University of Debrecen, Hungary
Abstract
There is a growing demand in the biopharmaceutical industry for rapid N-glycosylation profiling of carbohydrates associated with therapeutic antibodies in all phases of biologics development. Glycan identification historically has been a time consuming method. Liquid phase and gel based glycoanalytical methods (e.g., HPLC, UHPLC, CE, and PAGE) generally require labor intensive, time consuming sample preparation including glycan release, purification, fluorophore labeling and pre-concentration prior to analysis. In addition, numerous centrifugation and vacuum-centrifugation steps were necessary—making full automation of such protocols complex and expensive.SCIEX has developed a Fast Glycan Labeling and Analysis Kit for high-speed sample preparation and analysis of N-linked oligosaccharides. This Technical Note demonstrates optimization of previously published work2 including all steps of the rapid magnetic bead based protocol.

Figure 1: The PA 800 Plus Pharmaceutical Analysis System with laser induced fluorescence detection (CE-LIF).
Introduction
SCIEX has developed a Fast Glycan Labeling and Analysis Kit for high-speed sample preparation and analysis of N-linked oligosaccharides.1 This assay utilizes a high resolution gel buffer to ensure excellent separation performance of target molecules. Glycan release, fluorophore labeling and clean-up all were optimized resulting in 1 hour sample preparation time using a novel magnetic bead mediated process—ensuring excellent yield and high reproducibility. Analysis occurred in 5 minutes, and included automated, immediate glycan identification.
Modifications include rapid endoglycosidase digestion and fluorophore labeling reaction time, optimized labeling temperature and rapid Capillary Electrophoresis (CE) separation using the PA800 Plus Pharmaceutical Analysis System (Figure 1). The procedure can be performed for as few as a single sample to as many as 96 samples at a time using automated liquid handling platforms without the need for centrifugation or vacuum centrifugation3 commonly used in other glycan sample preparation methods. Capillary electrophoresis separation with laser-induced fluorescence detection (CE-LIF) of the fluorophore labeled glycans is also optimized to provide rapid and high resolution separations of the labeled sugars in 5 minutes, enabling rapid analysis in both the manual and automated sample preparation processes.
Features of the Fast Glycan Labeling and Analysis Kit
- Utilizing a novel magnetic-bead mediated sample preparation protocol, small numbers of samples can be prepared manually in microvials or up to 96 samples can be processed in a microtiter plate format using automated liquid handling in 1 hour without the requirement of centrifugation or vacuum-centrifugation.
- Fast analysis of the resulting fluorophore labeled glycans can be performed in 5 minutes per sample using the PA 800 Plus Pharmaceutical Analysis System with laser induced fluorescent detection.
- The 32 Karat GU Value Software Component automatically generates a report identifying all carbohydrate species separated with glycan image and monoisotopic mass information.
- This protocol has been tested on 96-well plate formats using a Laboratory Automation Workstation for preparation of immunoglobulin G samples.
Methods
Individual steps of the manual and automated approaches of the magnetic bead mediated sample preparation protocol were performed using the SCIEX Fast Glycan Labeling and Analysis Kit. (P/N B94499PTO, Figure 2). PNGase F was used for enzymatic release of the N-linked carbohydrates, followed by derivatization with a charged fluorophore (aminopyrene trisulfonate, APTS). The resulting fluorophore-labeled glycans were separated and analyzed by CE using a PA 800 Plus Pharmaceutical Analysis System equipped with a solid state LIF detector (488 nm excitation, 520 nm emission) (SCIEX, Brea, CA).
Separation was performed using a pre-built bare fused silica capillary cartridge (20 cm effective and 30 cm total capillary length, 50 μm I.D., P/N A55625) employing a novel, high resolution separation gel buffer (HR-NCHO). The three internal GU standards for instant, automated structural assignment are included in the kit.4 The applied voltage was 30 kV and the separation temperature was actively thermostatted at 25° C by the liquid cooling system of the unit. Electric field mediated sample introduction was used at 2 kV for 2 seconds. CE data was acquired and analyzed using the 32 Karat™ software package ver10.1 (SCIEX, Brea, CA) including the GU Value Software Component to generates a comprehensive report identifying all these glycan species separated along with their image and monoisotopic mass information.
Figure 2: The PA 800 Plus Fast Glycan Labeling and Analysis Kit with high resolution separation gel buffer (HR-NCHO).
Results and discussion
Our results illustrate that by applying the Fast Glycan Labeling and Analysis Kit Assay for N-glycosylation analysis, sample preparation can be completed in as fast as 1 hour with excellent yield and high level of reproducibility. Because the centrifugation steps during the sample preparation protocol are unnecessary, the process can be automated using liquid handling robots to provide a flexible and robust approach capable of reproducible sample processing. The Laboratory Automation Workstation offered fast sample preparation with minimized contamination risks.
Fluorophore labeled N-glycans were then analyzed by CE using an optimized rapid separation method for a single sample (Figure 4) or in 96-well plate formats (Figure 5). High resolution N-glycan separation for each sample was obtained in approximately 5 minutes. Our novel triple-internal standard approach along with the GU Value Software Component facilitates automated glycan identification.4 Table 1 delineates the abbreviated names of the structures and the corresponding GU units of IgG N-glycans of interest as well as their monoisotopic masses. The combination of rapid glycan sample preparation (release and labeling). fast, high resolution separation and automated glycan identification enables data analysis in as little as 75 min for a single sample. This workflow enhancement provides the means to incorporate glycan analysis more readily into process and development practices to easily obtain required information.
In summary, the PA 800 Plus Pharmaceutical Analysis System with the new Fast Glycan Labeling and Analysis Kit provides fast, robust sample preparation and separation with automated glycan identification for comprehensive N-glycosylation analysis of IgG molecules.
Figure 4: Ultrafast high resolution CE analysis of PNGase F released and fluorophore labeled IgG N-glycans using the HR-NCHO gel buffer. Abbreviated names of the structures (Table 1) were assigned using a triple-internal standard in combination with the GU Value Software Component and a comprehensive database.
Table 1: Abbreviated names of the structures and the corresponding GU units of IgG N-glycans of interest using the Fast Glycan Labeling and Analysis Kit with the triple-internal standard feature and the GU Value Software Component.
Figure 5: 96 sample batch processing of released and fluorophore labeled IgG N-glycans using the PA 800 Plus Pharmaceutical Analysis System.
References
- Fast Glycan Labeling and Analysis Application Guide, #B94499
- Cs.Varadi, C.Lew, A.Guttman, Rapid magnetic bead based sample preparation for automated and high throughput N-glycan analysis of therapeutic antibodies, Anal. Chem. 86 (2014) 5682-5687.
- M.Szigeti, C.Lew, K.Roby, A.Guttman, Fully automated sample preparation with ultrafast N-glycosylation analysis of therapeutic antibodies, JALA 21 (2016) 281-286.
- G.Jarvas, M.Szigeti,J.Chapman, A.Guttman, Triple-internal standard based glycan structural assignment method for capillary electrophoresis analysis of carbohydrates, Anal. Chem. 88 (2016) In Press.
- Zs.Kovacs, M.Szarka, M.Szigeti, A.Guttman, Separation window dependent multiple injection (SWDMI) for large scale analysis of therapeutic antibody N-glycans, J. Pharm. Biomed. Analysis, 128 (2016) 367-370.
Acknowledgements
The authors acknowledge the support of the MTA-PE Translational Glycomics project (#97101).
 Click to enlarge
Click to enlarge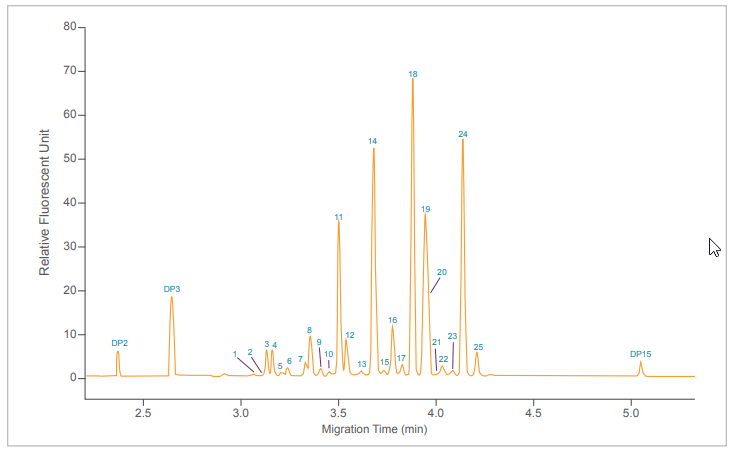 Click to enlarge
Click to enlarge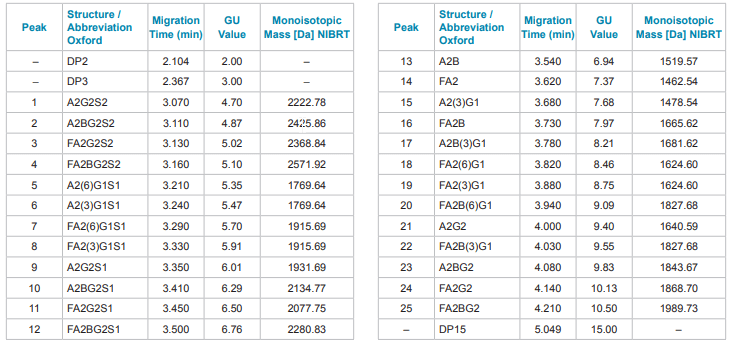 Click to enlarge
Click to enlarge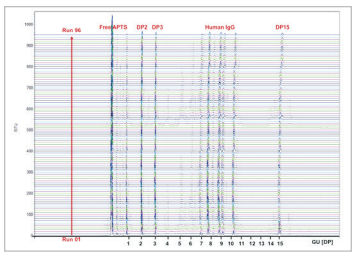 Click to enlarge
Click to enlarge