Automated solid-phase extraction system for the analysis of PFAS in drinking water
Craig M. Butt1, Lily Sanchez2, Prem Parmar2, Sam Lodge3
1 SCIEX, USA; 2 Orange County Water District, Fountain Valley, CA; 3 Phenomenex, USA
Abstract
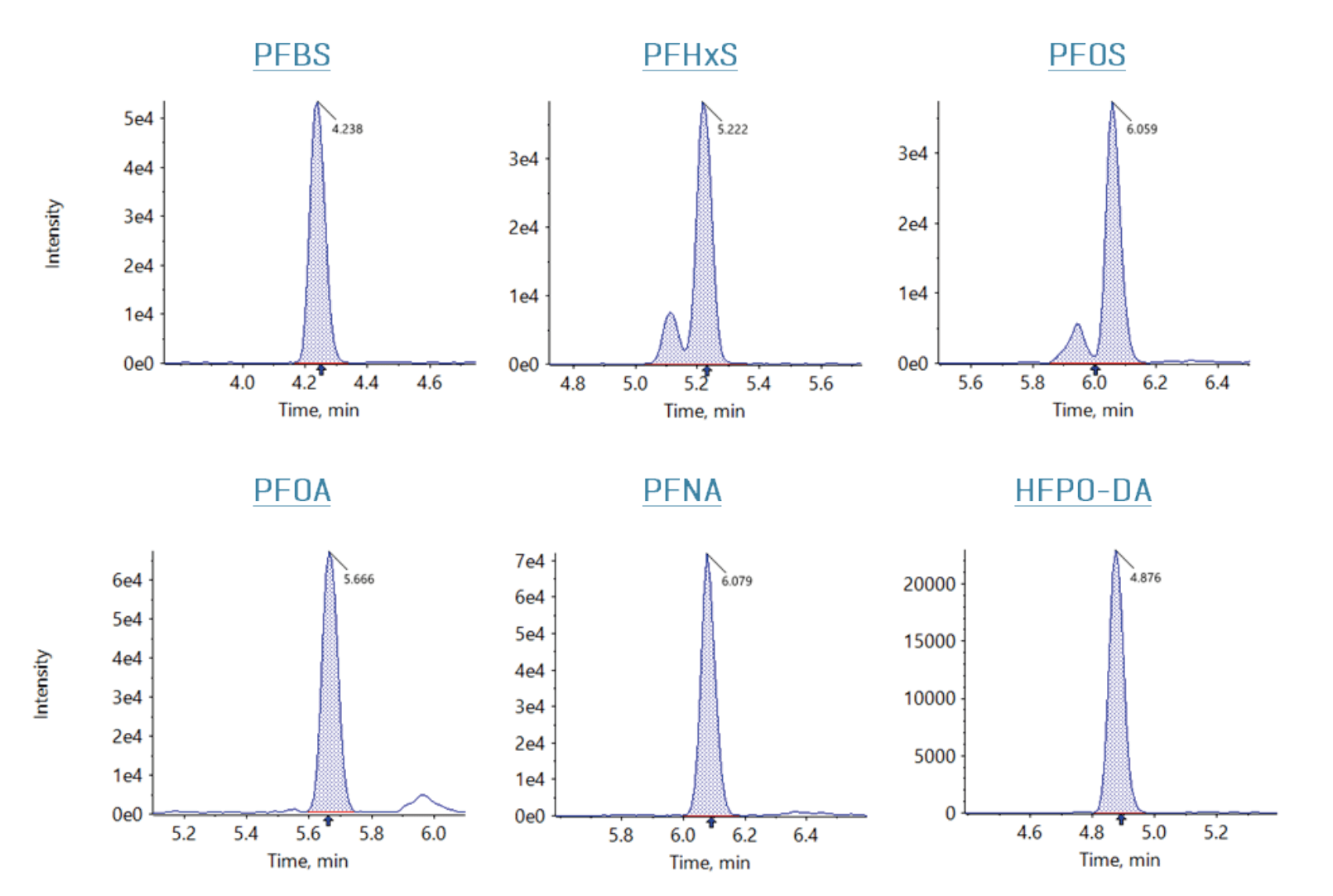
This technical note demonstrates the use of the PromoChrom automated solid-phase extraction (SPE) system for the analysis of PFAS in drinking water following EPA Method 533. Using the SCIEX QTRAP 6500+ system, negligible background contamination was observed as well as excellent method performance in spikes into reagent water and raw untreated groundwater samples. Milli-Q water blank spikes at 2, 40 and 70 ng/L showed mean recovery ranging from 96% to 115% with the mean precision <10% coefficient of variation (CV). Sample matrix and duplicate spikes at 2 and 40 ng/L showed mean recovery between 95% and 122% with <13%CV (representative chromatograms shown in Figure 1). Analysis of raw untreated groundwater samples showed the detection of 5 out of the 6 EPA-regulated PFAS compounds.
Figure 1. Representative extracted ion chromatograms (XICs) in a 2 ng/L laboratory fortified sample matrix (LFSM) sample for the 6 EPA-regulated PFAS compounds. Raw, untreated groundwater was used for the LFSM sample.
Key benefits of the PromoChrom automated SPE system for the analysis of PFAS in drinking water
- Automated sample extraction. Reduced sample preparation time through the fast, reproducible extraction and clean-up of drinking water samples
- Low system background contamination. Quality control blanks predominantly showed non-detectable PFAS levels
- Excellent method performance in quality control spikes. Method performance was evaluated with spikes into Milli-Q water and raw untreated groundwater samples
- Real-world water samples. 10 raw untreated groundwater samples analyzed to demonstrate method applicability
Introduction
The contamination of PFAS in drinking water is well-known, widespread and global in nature.1-3 PFAS drinking water levels are formally regulated, or under proposed regulations, in several countries and regions, including the United States4 and the European Union.5 In the United States, the Environmental Protection Agency (EPA) has promulgated several methods for PFAS analysis in drinking water (EPA 537, 537.1, 533) as well as non-potable water samples such as surface water, groundwater and wastewater (EPA 1633, 1633A). The EPA methods utilize solid-phase extraction (SPE) for sample concentration and clean-up. Traditionally, the SPE procedure has been manually performed with vacuum manifolds, which is time-intensive and occasionally error-prone. However, in recent years automated SPE systems have become commercially available with the aim of increasing throughput, reducing manual effort and improving reproducibility. These systems can extract multiple samples in parallel, following an EPA-compliant method, and can be modified to reduce fluoropolymer components, reducing background PFAS levels. In this technical note, a method is described for PFAS analysis in drinking water following EPA Method 533, using the PromoChrom SPE-03 automated SPE system and the SCIEX QTRAP 6500+ system.
Methods
Samples and reagents: Calibration standards were purchased from Phenova (Golden, Colorado) and Absolute Standards (Hamden, CT). Stable-isotope labelled standards were purchased from Wellington Laboratories (Guelph, Ontario).
Quality control (QC) samples: The method performance was evaluated using 3 types of QCs, laboratory reagent blank (LRB), laboratory fortified blank (LFB) and, laboratory fortified sample matrix (LFSM) and duplicate (LFSMD) samples. LRB samples (n=38) consisted of Milli-Q water spiked with the isotope dilution analogues (IDAs) and isotope performance standards (IPSs) only. LFBs were comprised of Milli-Q water spiked with the target analytes, IDAs and IPSs. LFB spikes were performed at 2 ng/L (n=38), 40 ng/L (n=38) and 70 ng/L (n=37). LFSM and LFSMD samples were from untreated groundwater samples spiked with the target analytes at 2 ng/L (n=13) and 40 ng/L (n=23), as well as the IDA and IPS mixes. All QC samples were extracted and instrumentally analyzed using the procedures described below.
Real-world groundwater samples: 10 raw untreated groundwater samples collected from the influent of a PFAS treatment facility were analyzed between February 2024 and January 2025. These samples represent source water that had not yet undergone treatment and do not represent the final treated drinking water delivered to the community. The purpose of analyzing the raw groundwater samples was to demonstrate the applicability of the PromoChrom extraction and instrumental analysis methods.
Sample preparation: All samples were extracted during May-September 2024 using the Promochrom SPE-03 automated SPE system with the MOD-004 (sample bottle rinsing) and MOD-005 (minimal Teflon option) modifications. The automated SPE procedure is outlined in Table 3. The sample volume was 250 mL, and the SPE cartridge was the Phenomenex Strata SBD-L (500 mg/5 mL, P/N: 8B-S014-HCH). QC samples were collected between May and September 2024 and were comprised of 38 laboratory reagent blanks (LRB), 38 laboratory fortified blanks (LFB) at 2, 40 and 70 ng/L, 13 laboratory fortified sample matrix (LFSM) and duplicate (LFSMD) samples at 2 ng/L, and 23 LFSM and LFSMD samples at 40 ng/L.
LC chromatography: Chromatographic separation was performed using an Agilent 1200 LC system. The analytical column was a Phenomenex Gemini C18 column (3 mm, 100 x 2 mm, P/N: 00D-4439-B0) and the delay column was a Phenomenex Luna C18(2) column (5 mm, 30 x 2 mm, P/N: 00A-4252-B0). Mobile phase A was water with 20mM ammonium acetate, and mobile phase B was methanol. The gradient conditions used are presented in Table 1. The flow rate was 430 mL/min, the injection volume was 5 µL, and the column oven was 40oC.
Table 1: Chromatographic gradient for the analysis of PFAS in drinking water.
Mass spectrometry: Samples were analyzed using the QTRAP 6500+ system with electrospray ionization operated in negative mode. Data was acquired using multiple reaction monitoring (MRM) modes using optimized source and gas conditions (Table 2) and compound-specific parameters (Table 4 in the Appendix).
Data processing: Data acquisition was performed using Analyst (version 1.7.2) and processed using MultiQuant (version 3.0.3).
Table 2: Source and gas parameters for the analysis of PFAS in drinking water using the QTRAP 6500+ system.
Table 3: Automated Promochrom extraction procedure for EPA Method 533.
System background contamination: Laboratory reagent blanks (LRB)
The extensive series of LRB samples (n=38) evaluated the contamination originating from the PromoChrom system during the sample preparation protocol. None of the targeted PFAS analytes were detected except for one sample that showed low 6:2 FTS levels (2.2 ppt). Figure 2 shows representative XICs for the 6 EPA regulated PFAS compounds in the LRBs. These results demonstrate the ability of the PromoChrom automated SPE system to generate extracts with very low background PFAS levels. This is critical for achieving the low parts-per-trillion (ppt) reporting levels necessary to meet the 2024 EPA drinking water maximum contaminant levels (MCLs): 4 ppt for PFOA and PFOS, 10 ppt for PFNA, PFHxS and HFPO-DA.
Method performance in quality control spikes: Fortified blank and matrix samples
Method performance was evaluated in two different types of QC samples: LFBs and LFSMs/LFSMDs. LFBs consisted of spiked Milli-Q water samples processed through the PromoChrom system and instrumentally analyzed. The LFBs were prepared at three spiking levels: 2 ng/L (n=38), 40 ng/L (n=38) and 70 ng/L (n=37). The EPA 533 criteria specify that the LFB recovery must be within 50-150% at spiking levels near the 2 ng/L method reporting limit (MRL) and within 70-130% for the higher spike levels. Considering all targeted PFAS analytes, the mean LFB recovery ranged from 99.5% to 115% for the 2 ng/L spikes, 95.7% to 105% for the 40 ng/L spikes and 95.6 to 103% for the 70 ng/L spikes. Figure 2 shows representative XICs for the 2 ng/L LFB spikes. Figure 3 shows the mean analyte recovery for each spiking level and associated %CV bars as well as the ±30% accuracy tolerance lines (red dashed line). The mean precision was <10%CV for all analytes across the 3 spiking levels.
Figure 2. Extracted ion chromatograms (XICs) in representative laboratory reagent blank and laboratory fortified blank (2 ng/L spike level) samples for the six EPA regulated PFAS compounds (PFBS, PFHxS, PFOS, PFOA, PFNA and HFPO-DA).
Figure 3. Mean PFAS recovery in laboratory fortified blank samples (n=38 for 2 ng/L and 40 ng/L spikes, n=37 for 70 ng/L spike). Error bars represent the coefficient of variation percentage (%CV)Z
The LFSM and LFSMD samples consisted of spiked untreated groundwater samples processed through the PromoChrom system and instrumentally analyzed. The corresponding unspiked matrix samples were also analyzed to correct for background PFAS levels present. The LFSM and LFSMD samples were prepared at 2 ng/L (n=13) and 40 ng/L (n=23). Similar to the LFB samples, the EPA 533 criteria specify 50-150% recovery at the 2 ng/L MRL and 70-130% recovery for higher spiking concentrations. Table 4 shows the mean analyte recovery and precision (%CV) for the LFSM and LFSMD samples. The mean recovery range in the 2 ng/L spikes was 98-122% and 97-118% for the LFSM and LFSMD samples. For the 40 ng/L spikes, the mean recovery range was 96-110% and 95-109% in LFSM and LFSMD samples. The mean precision was <13%CV for all analytes in the 2 spiking levels. The mean relative percent difference (RPD) for the duplicate samples was 7% and 6% in the 2 ng/L and 40 ng/L spikes, respectively. All RPDs were within the EPA 533 criteria of <50% for the MRL spike and <30% for higher-level spikes.
Overall, the results from both the fortified reagent blank (LFBs) and sample matrix and duplicate (LFSMs/LFSMDs) samples demonstrate the ability of the PromoChrom automated SPE system and QTRAP 6500+ system to generate excellent quantitative data for PFAS in drinking water. Excellent recovery and precision were observed at both the MRL and higher levels.
Table 4: Mean recovery (%) and precision (%CV) in the laboratory fortified sample matrix (LFSM) and duplicates (LFSMD) for 2 ng/L (n=13) and 40 ng/L (n=23) spikes.
Real-world samples
The PromoChrom automated SPE sample preparation method was applied to raw untreated groundwater samples collected from February 2024 to January 2025 and analyzed using the 6500+ system. Concentrations were lowest for PFNA (range: 2.1-3.3 ng/L) and highest for PFOS (range: 54.7-73.7 ng/L) while HFPO-DA levels were <MRL. See Table 5 for the full data set. The samples were collected prior to treatment, and after undergoing treatment, all samples were non-detect for all PFAS analytes.
Table 5. PFAS concentrations for the 6 EPA-regulated compounds in raw untreated groundwater samples collected between February 2024 and January 2025.
Conclusions
- Automated sample preparation using the PromoChrom SPE system produced drinking water extracts with low background contamination, satisfactory to meet EPA maximum contaminant levels (MCLs) for PFAS
- Excellent PFAS recovery and precision in QC spike samples, meeting data quality requirements in EPA Method 533 using the SCIEX 6500+ system
References
- Ackerman Grunfeld, D.; Gilbert, D.; Hou, J. et al. Underestimated burden of per- and polyfluoroalkyl substances in global surface waters and groundwaters. Nat. Geosci. 2024, 17, 340-346. DOI: 10.1038/s41561-024-01402-8.
- Smalling, K.L.; Romanok, K.M.; Bradley, P.M. et al. Per- and polyfluoroalkyl substances (PFAS) in United States tapwater: Comparison of underserved private-well and public-supply exposures and associated health implications. Environ. Int. 2023, 178, 108033. DOI: j.envint.2023.108033.
- European Environment Agency. PFAS pollution in European waters. December 9, 2024. https://www.eea.europa.eu/en/analysis/publications/pfas-pollution-in-european-waters (accessed 2025-02-10).
- United States Environmental Protection Agency. Per- and polyfluoroalkyl substances (PFAS): Final PFAS national primary drinking water regulation. https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (accessed 2025-02-10).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. http://data.europa.eu/eli/dir/2020/2184/oj (accessed: 2025-02-10).
Table 4: Compound-specific MRM parameters for the analysis of PFAS in drinking water by EPA Method 533 using the QTRAP 6500+ system.
 Click to enlarge
Click to enlarge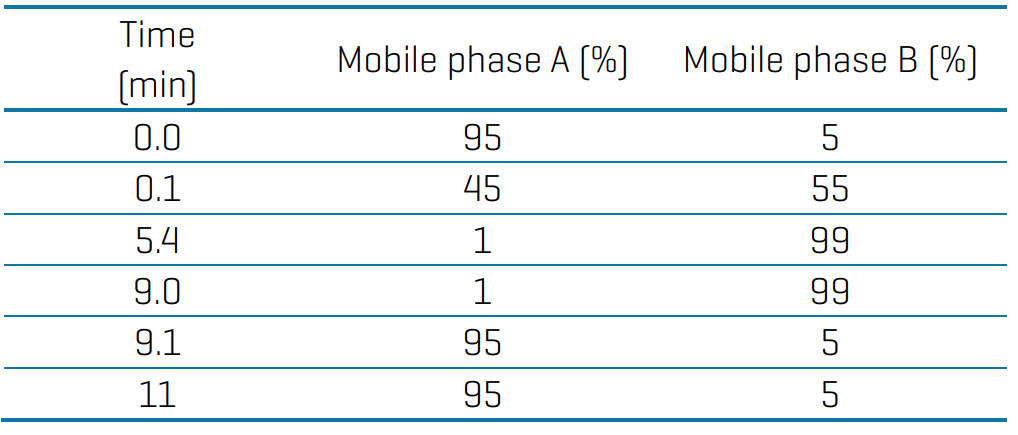 Click to enlarge
Click to enlarge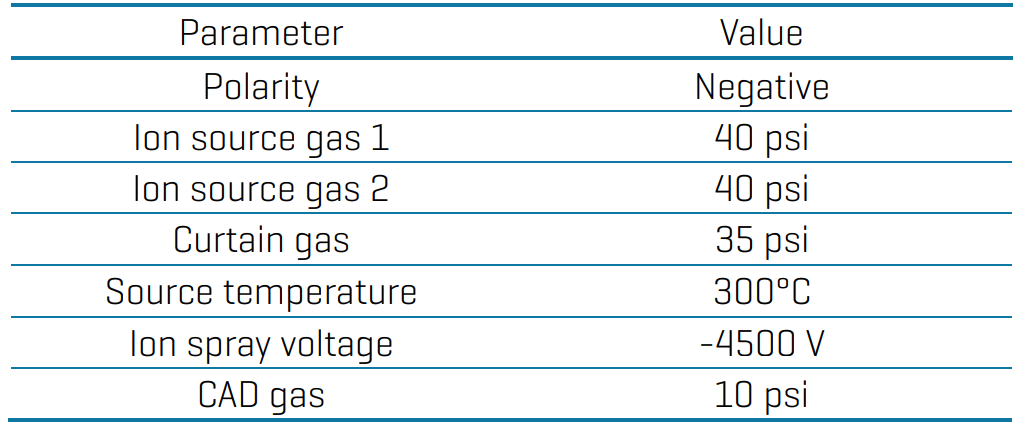 Click to enlarge
Click to enlarge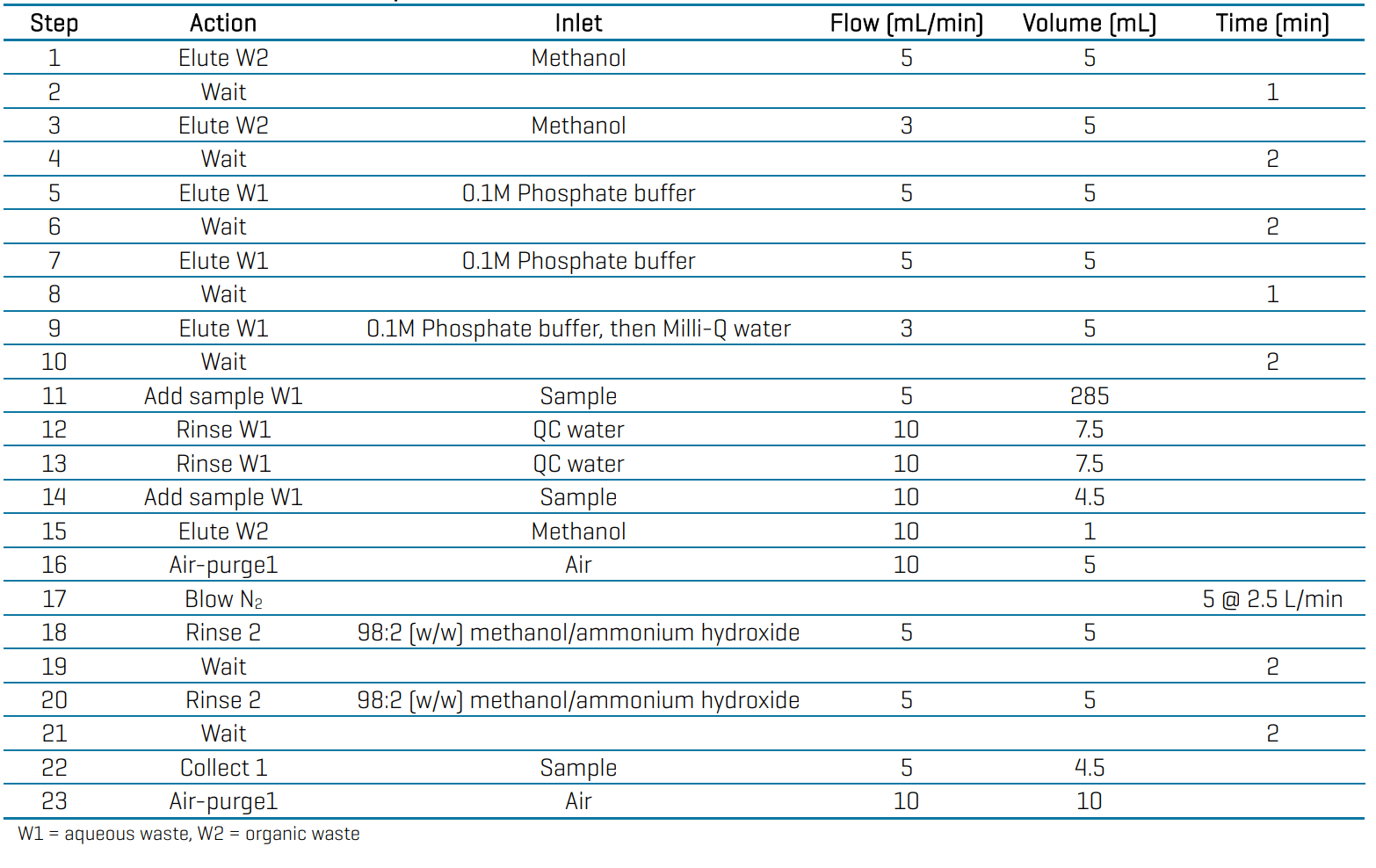 Click to enlarge
Click to enlarge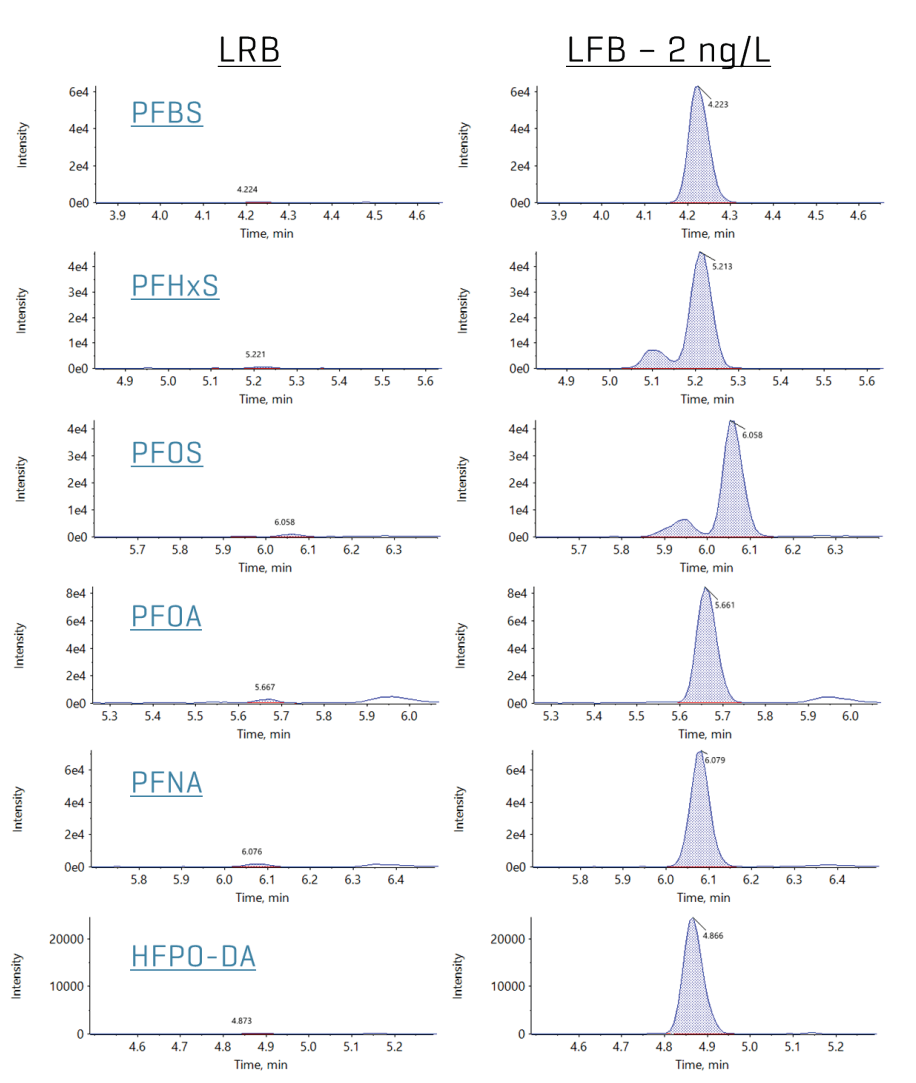 Click to enlarge
Click to enlarge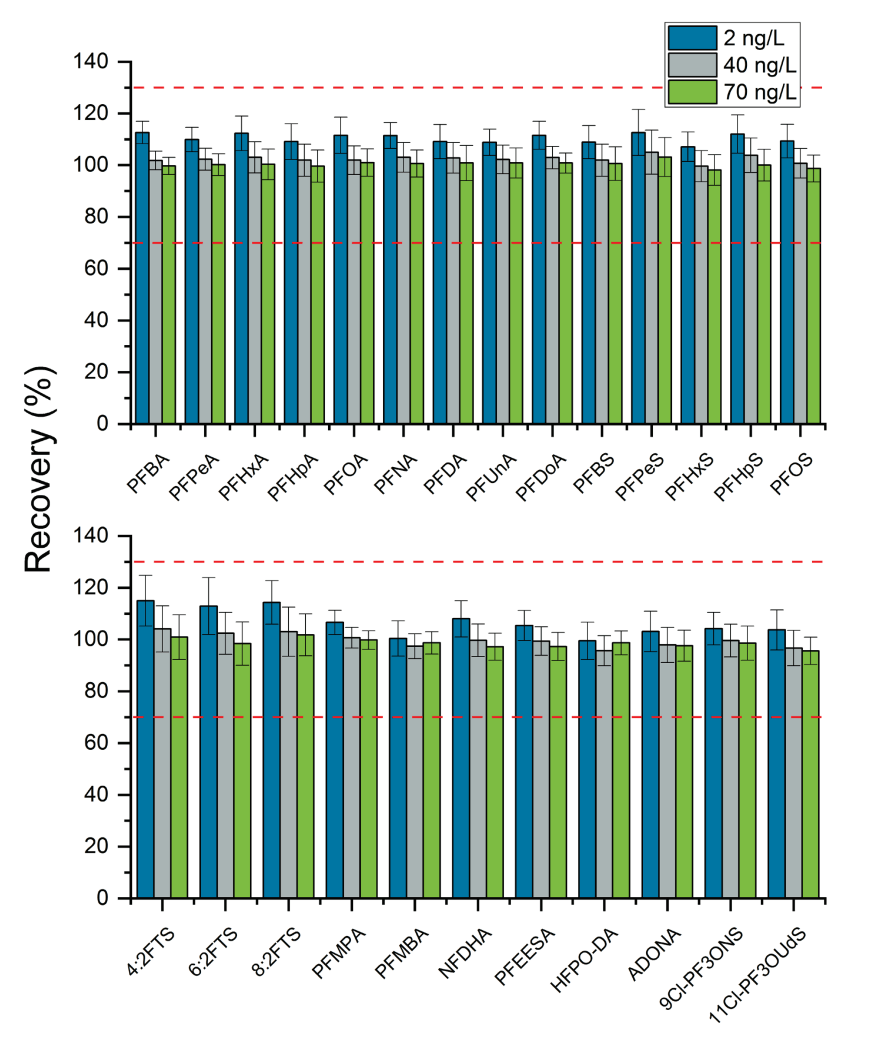 Click to enlarge
Click to enlarge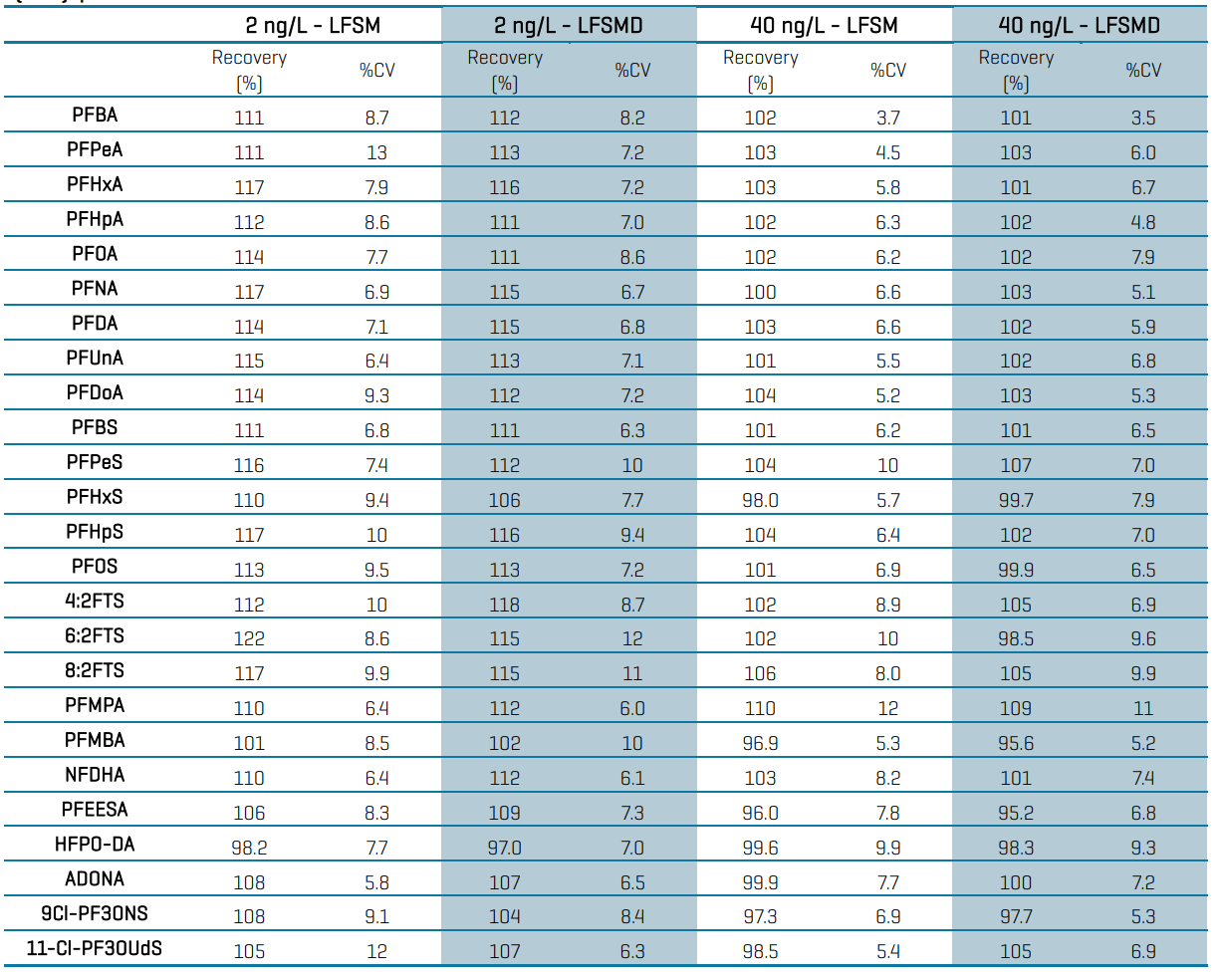 Click to enlarge
Click to enlarge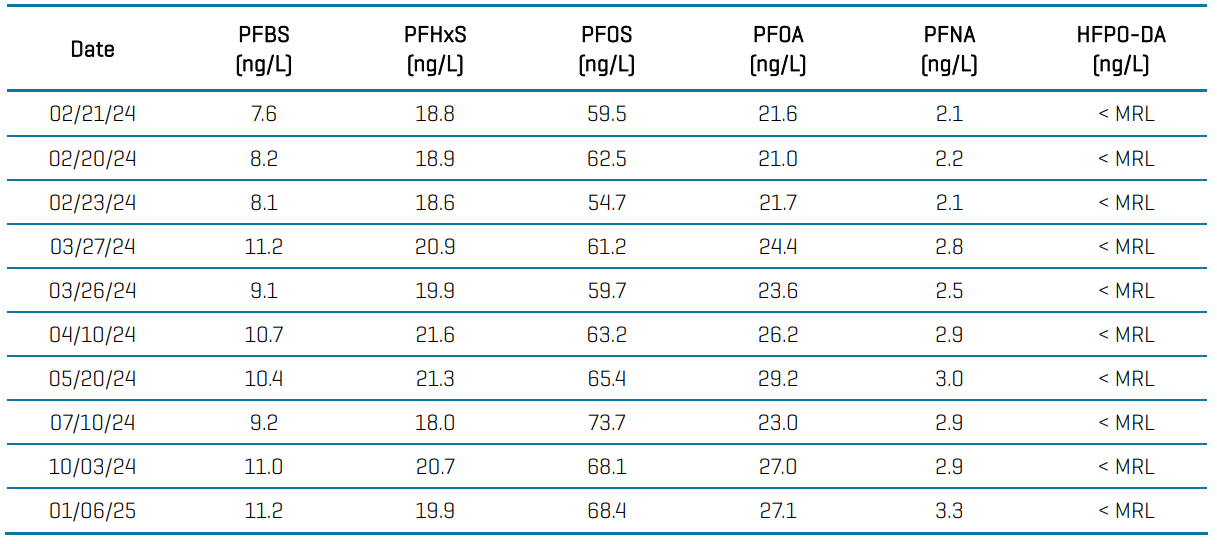 Click to enlarge
Click to enlarge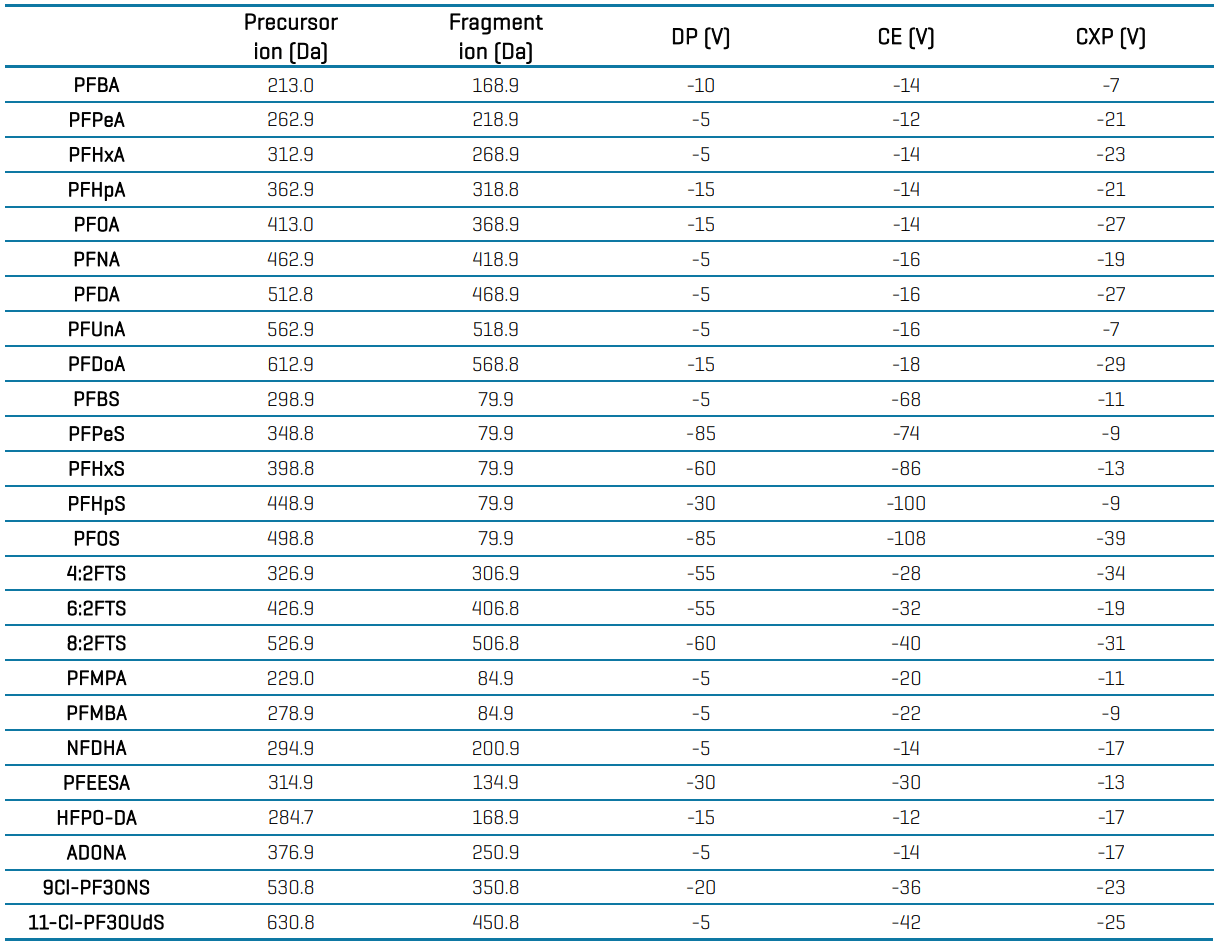 Click to enlarge
Click to enlarge