Nontargeted and suspect screening of per- and polyfluoroalkyl substances (PFAS) in food contact materials
Holly Lee1; Craig M. Butt2
1SCIEX, Canada; 2SCIEX, USA
Abstract
This technical note demonstrates the nontargeted and suspect screening for PFAS in food contact materials (FCMs) using SWATH data-independent acquisition (DIA) on the X500R QTOF system. Comprehensive MS/MS coverage from SWATH DIA enabled nontargeted and suspect screening for unknown and PFAS previously reported in FCMs. Several classes of PFAS comprised of precursors, degradation intermediates and terminal metabolites, were identified at varying levels of confidence, including a legacy compound that had been phased out in commercial products since the early 2000s.1
Introduction
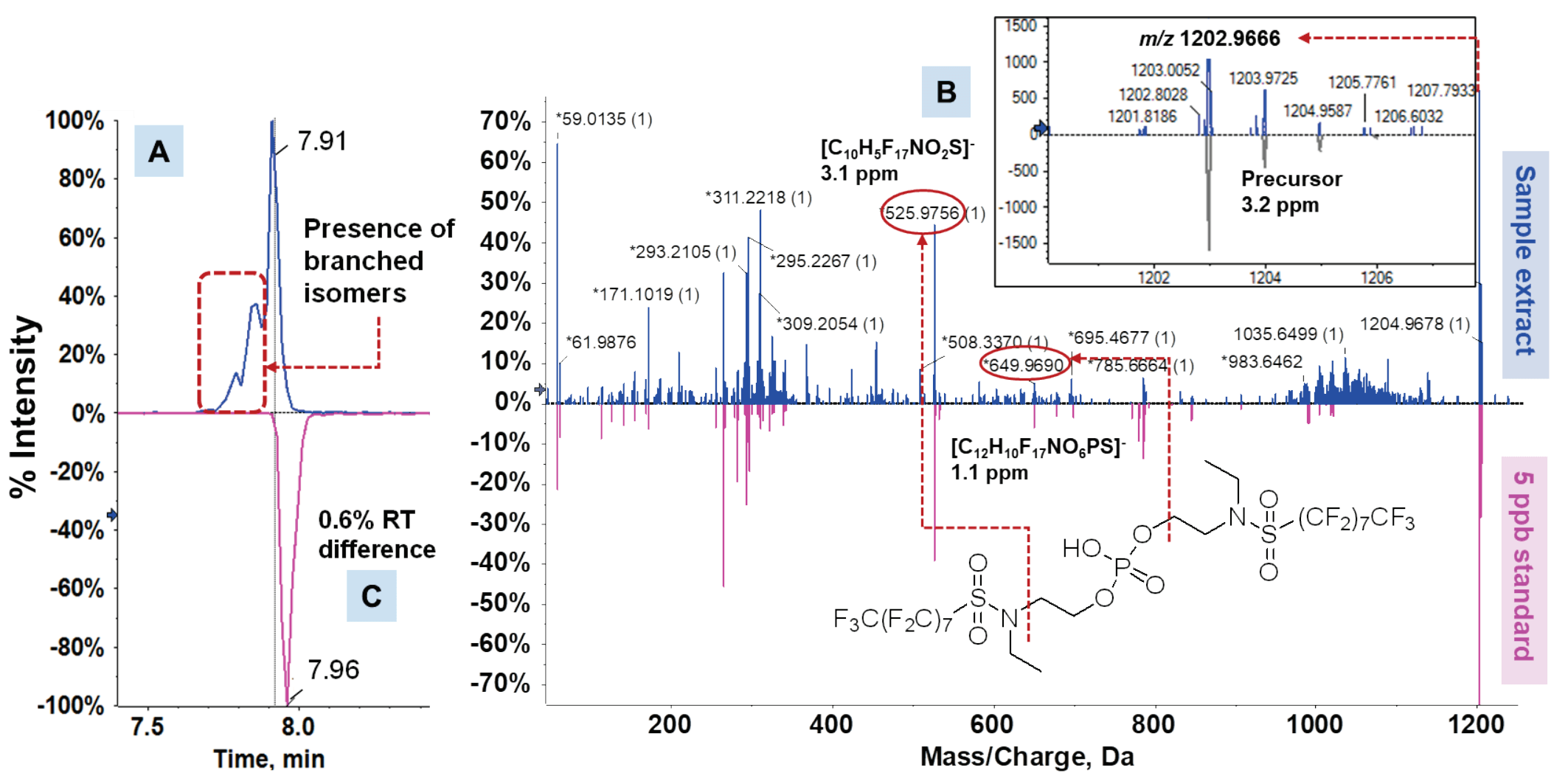
Due to their applications as grease proofing agents and dispersion aids in ink labels, PFAS have been widely reported in FCMs.2-5 However, targeted analysis typically accounts for a minor proportion of the total fluorine burden in FCMs, while the remainder is largely dominated by non-extractable fluoropolymers, legacy and replacement substances and transformation products. Nontargeted acquisition (NTA) using accurate mass spectrometry enables the screening of unknown PFAS without a priori knowledge. This technique has led to the discovery of >750 PFAS in a variety of environmental matrices.6 Here, an end-to-end workflow using SWATH DIA for non-targeted feature finding and suspect screening for prospective PFAS in FCMs is described. Figure 1 shows the identification of perfluorooctanesulfonamidoethanol phosphate diester (diSAmPAP) in an egg carton, a chemical that was used as a paper protectant in FCMs prior to its commercial phase-out.
Figure 1. Identification of a legacy PFAS in an egg carton composed of recycled paper. Nontargeted processing of SWATH DIA data revealed a feature (A) that was identified as diSAmPAP by suspect screening based on isotope pattern match and diagnostic fragments with good mass error (<5 ppm) (B). Identification was further confirmed by using a reference standard (C) to authenticate retention times (RTs).
Key benefits for the identification of PFAS in FCMs using the X500R QTOF system
- Comprehensive MS/MS coverage: SWATH DIA acquisition enabled nontargeted and suspect screening for both known and unknown PFAS
- Confident identification: Compound identification was supported by mass accuracy, isotope pattern match, RT confirmation against available standards and spectral MS/MS matching against published or library reference spectra
- Easy software tools for data filtering: Custom calculations and metric plots in SCIEX OS software enabled common data reduction strategies for identifying prospective PFAS features from NTA, such as background signal removal, Kendrick mass defect filtering and homologous series searching
Experimental methods
Chemicals and samples: All PFAS standards and their corresponding mass-labeled internal standards were purchased from Wellington Laboratories. FCM samples were collected from local retailers and restaurants during 2023 in Toronto, Ontario, Canada. Samples included take-out containers comprised of varying materials, such as molded fiber, polystyrene foam, compostable, and recyclable plastic; pastry bags and liners, plastic fruit bags; beverage containers; chocolate and candy bags, parchment paper, a microwaveable popcorn bag, pizza liners and an egg carton.
Sample preparation: The FCM samples were cut into small pieces ranging from 0.2 to 1.5 g. In a polypropylene tube, each sample was spiked with a mixed internal standard solution. After adding 15 mL of methanol, the sample was shaken vigorously for 1 hour at 40oC, sonicated for an additional hour and centrifuged for 10 minutes. The supernatant was transferred to a clean polypropylene tube, evaporated to near dryness under nitrogen gas and reconstituted in 0.5 mL of 80:20 (v/v) methanol/aqueous mobile phase for LC-MS/MS analysis. Methanol was also extracted as procedural blanks.
Chromatography: LC separation was performed on a SCIEX ExionLC AC system using a Phenomenex Luna Omega PS C18 as the analytical column (100 x 2.1 mm, 3 µm, P/N 00D-4758- AN) and Phenomenex Luna C18(2) as the delay column (50 x 4.6 mm, 5 µm, P/N 00B-4252-EO). A flow rate of 0.6 mL/min, an injection volume of 10 µL and a column temperature of 30ºC were used. The LC conditions used are shown in Table 1.
Table 1. Chromatographic gradient.
Mass spectrometry: Analysis was performed using the X500R QTOF system in negative electrospray ionization mode. Table 2 lists the source and gas conditions used, while Table 3 lists the parameters for SWATH DIA. The SWATH DIA method consisted of 17 TOF MS/MS windows of variable size, each with an accumulation time of 50 msec, over a precursor mass range of 45–1250 Da.
Table 2. Source, gas and temperature conditions.
Table 3. SWATH DIA conditions.
Data processing: Data were acquired and processed using SCIEX OS software, versions 3.1.6 and 3.3. The SCIEX Fluorochemical HR-MS/MS Spectral Library 2.0 was used for library searching.
Nontargeted screening for PFAS in FCM extracts
One of the main challenges with NTA is distinguishing compounds of interest from the hundreds to thousands of features typically present in full scan TOF MS data. This is particularly challenging for low-level compounds such as PFAS. Figure 2 shows a workflow comprised of data reduction and filtering techniques in SCIEX OS software that can help prioritize features over the background.
Figure 2. Nontargeted discovery of 6:2 diPAP in a recyclable plastic container. A) Overlaid total ion chromatograms (TIC) demonstrated the presence of many potential PFAS compounds in the FCM extract, as compared to the procedural blank. B) Data reduction strategies like blank and KMD filtering accelerated the process of targeting features that were uniquely present in the FCM sample and structurally similar to PFAS. C) Homologous series searching in KMD plots visually identified prospective PFAS features, followed by D) compound identification based on low mass error (ppm), isotope pattern, (E) MS/MS library match and RT match against authentic standards, if available.
Data filtering by eliminating common features between unknown and blank samples can be achieved by a blank subtraction workflow in SCIEX OS software.7 The peak area ratio from features present in both the unknown FCM and blank samples was calculated (Figure 2B). An area ratio threshold of 10 was applied to isolate unique features in the samples from background signals in solvent, laboratory and procedural blanks.
Highly fluorinated compounds such as PFAS tend to have low or negative mass defects,6 which represent the difference between the exact and nominal mass of the molecule. However, filtering on these low to negative mass ranges can result in false positives due to the presence of other heteroatom-containing or halogenated compounds with similar mass defects. A more specific technique for flagging prospective PFAS features in NTA is homologous series searching based on repeating subunits that are uniquely present in the chemical structures of PFAS. For example, -CF2- and -CF2CF2- are repeating units commonly found in the fluoroalkyl tails of PFAS. Here, the Kendrick mass (KM) and Kendrick mass defect (KMD) are calculated by normalizing the experimentally observed mass of a compound by the integer mass of a repeating subunit, as shown by equations 1 and 2.8 In SCIEX OS software, these calculations are natively performed in the results and facilitated by functions such as ROUND to derive the nominal mass (Figure 2B).
Homologous compounds with the same core structure but different numbers of repeating subunits can be easily identified in KMD plots by those horizontally aligned on the same KMD value. Figure 2C shows an example of a homologous series of 4 features spaced equally apart by m/z of 100 in a KMD plot for a recyclable plastic container extract. A homolog m/z spacing of 100 indicates that the compounds differ in repeating units of CF2CF2 in their structures, which is consistent with the mixture of chain lengths produced from the manufacture of fluorotelomer-based chemicals. The same RT shift was also observed between these homologs, which is consistent with the theoretical chromatographic pattern in increasingly long-chain PFAS.
Upon identifying the first homolog as 6:2 polyfluoroalkyl phosphate diester (diPAP), the remaining three were easily deciphered as the longer-chained 6:2/8:2, 8:2 and 8:2/10:2 analogs (Figures 2D and 2E). DiPAPs are known to be used as grease proofing agents in FCMs. Due to the lack of a reference standard, the 8:2/10:2 diPAP could only be identified with level 2a confidence. These levels are associated with criteria such as mass error, isotopic pattern match and RT match against a standard for rating the confidence in the PFAS identification.9
Suspect screening and compound confirmation of identified PFAS
From the list of prospective PFAS features returned from the nontargeted workflow, further screening based on a suspect list of PFAS previously reported in FCMs revealed the detection of several short-chain PFCAs (≤C7), 6:2 FTCA, 6:2 FTUCA and several fluorinated phosphate esters (Table 4). Compound confirmation was based on a low mass error of <5 ppm for the precursor and fragment m/z peaks, isotope ratio pattern, spectral MS/MS matching and RT matches against authentic standards, if available. Each identification was assigned a confidence level based on how many of these criteria were met.9
The presence of perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), the 6:2 fluorotelomer saturated (FTCA) and unsaturated acids (FTUCA) is consistent with their previous detections in FCMs.2-5 Both the 6:2 FTCA and 6:2 FTUCA are known transformation intermediates of 6:2 fluorotelomer-based precursors and can be further converted into PFCAs.10 Previous observations of their concentrations increasing in FCMs after 2 years of storage suggested the presence of fluorotelomer-based precursors like the diPAPs.3
In addition to the recyclable plastic take-out container, diPAPs were also detected in a chocolate cardboard box and an egg carton, both composed of recycled molded fiber. This suggests recycled paper may be a sink for diPAPs from multiple paper sources that may originally contain these chemicals. In these same 2 samples, diSAmPAP was also identified with low mass error, isotope pattern match, MS/MS fragments and RT matches against a reference standard (Figure 1). Similar to previous reports of the branched diSAmPAP isomers in soil,5 the detection observed here can only be verified with level 1b confidence due to the discrepancy in the chromatographic peak against the linear standard used. The diSAmPAPs were historically used as FCM components until the phase-out of perfluorooctylsulfonyl (POSF)- based chemistries in North America in 2002.1
Table 4. Examples of several PFAS identified in 6 FCM extracts using suspect screening. Compound identification was based on precursor and fragment mass error, RT matches against authentic standards (if available), MS/MS diagnostic fragments and library match (if available) with the confidence level assigned.
Conclusion
- SWATH DIA acquisition on the X500R QTOF system provided comprehensive MS/MS fragmentation fingerprints that enabled nontargeted and suspect screening of PFAS in FCMs
- Nontargeted and suspect screening identified PFAS that are not typically monitored in targeted analysis like the diPAPs and diSAmPAP with level 1a and 1b confidence based on mass accuracy, isotope ratio pattern, spectral MS/MS matches and RT matches against authentic standards
- Software features in SCIEX OS such as custom calculations and metric plots, facilitated an NTA workflow comprised of blank subtraction, KMD filtering and homologous series searching for prospective PFAS identification
References
- Phase-out Plan for POSF-based Products; U.S. EPA Public Docket AR226-0600, OPPT-2002-0043; 3M Specialty Materials Markets Group: St. Paul, MN, 2000.
- Sapozhnikova, Y. et al. Assessing per- and polyfluoroalkyl substances in globally sourced food packaging. Chemosphere. 2023, 337, 139381.
- Schwartz-Narbonne, H. et al. Per- and Polyfluoroalkyl Substances in Canadian Fast Food Packaging. Environ. Sci.: Technol. Lett. 2023, 10, 343-349.
- Straková, J. et al. Throwaway Packaging, Forever Chemicals: European wide survey of PFAS in disposable food packaging and tableware. 54 p.
- Bugsel, B. et al. LC-HRMS screening of per- and polyfluorinated alkyl substances (PFAS) in impregnated paper samples and contaminated soils. Anal. Bioanal. Chem. 2021, 414, 1217-1225.
- Liu, Y. et al. High-resolution mass spectrometry (HRMS) methods for nontarget discovery and characterization of poly- and per-fluoroalkyl substances (PFASs) in environmental and human samples. TrAC Trends Anal. Chem. 2019, 121, 115420.
- Oetjen, K.A. Nontarget and suspect screening analysis of samples containing compounds derived from tire rubber. SCIEX technical note, RUO-MKT-02-13659-A.
- Bugsel, B. et al. LC-MS screening of poly- and perfluoroalkyl substances in contaminated soil by Kendrick mass analysis. Anal. Bioanal. Chem. 2020, 412, 4797-4805.
- Charbonnet, J.A. et al. Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High- Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2022, 9, 473-481.
- Lee, H. et al. Biodegradation of Polyfluoroalkyl Phosphates as a Source of Perfluorinated Acids to the Environment. Environ. Sci. Technol. 2010, 44, 3305-3310.
 Click to enlarge
Click to enlarge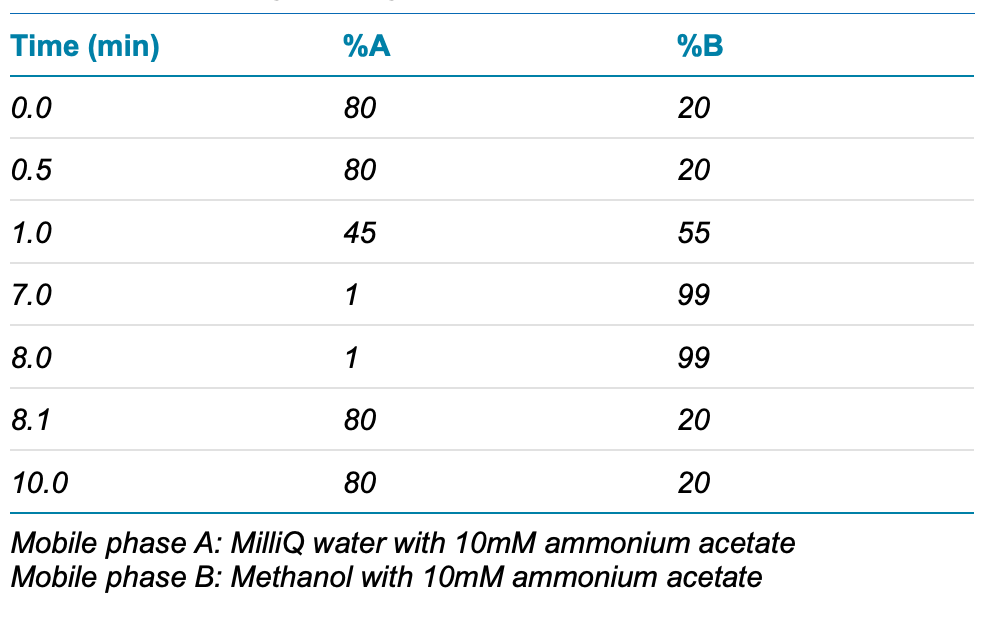 Click to enlarge
Click to enlarge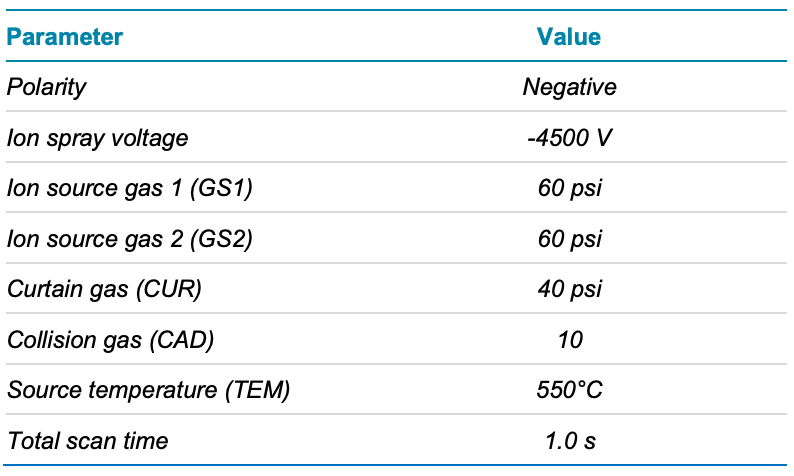 Click to enlarge
Click to enlarge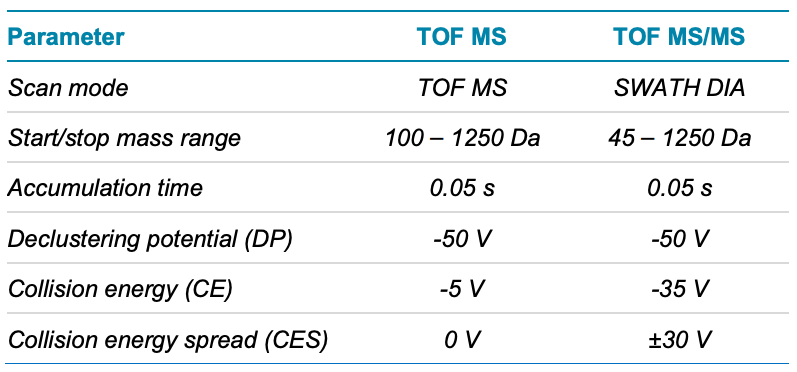 Click to enlarge
Click to enlarge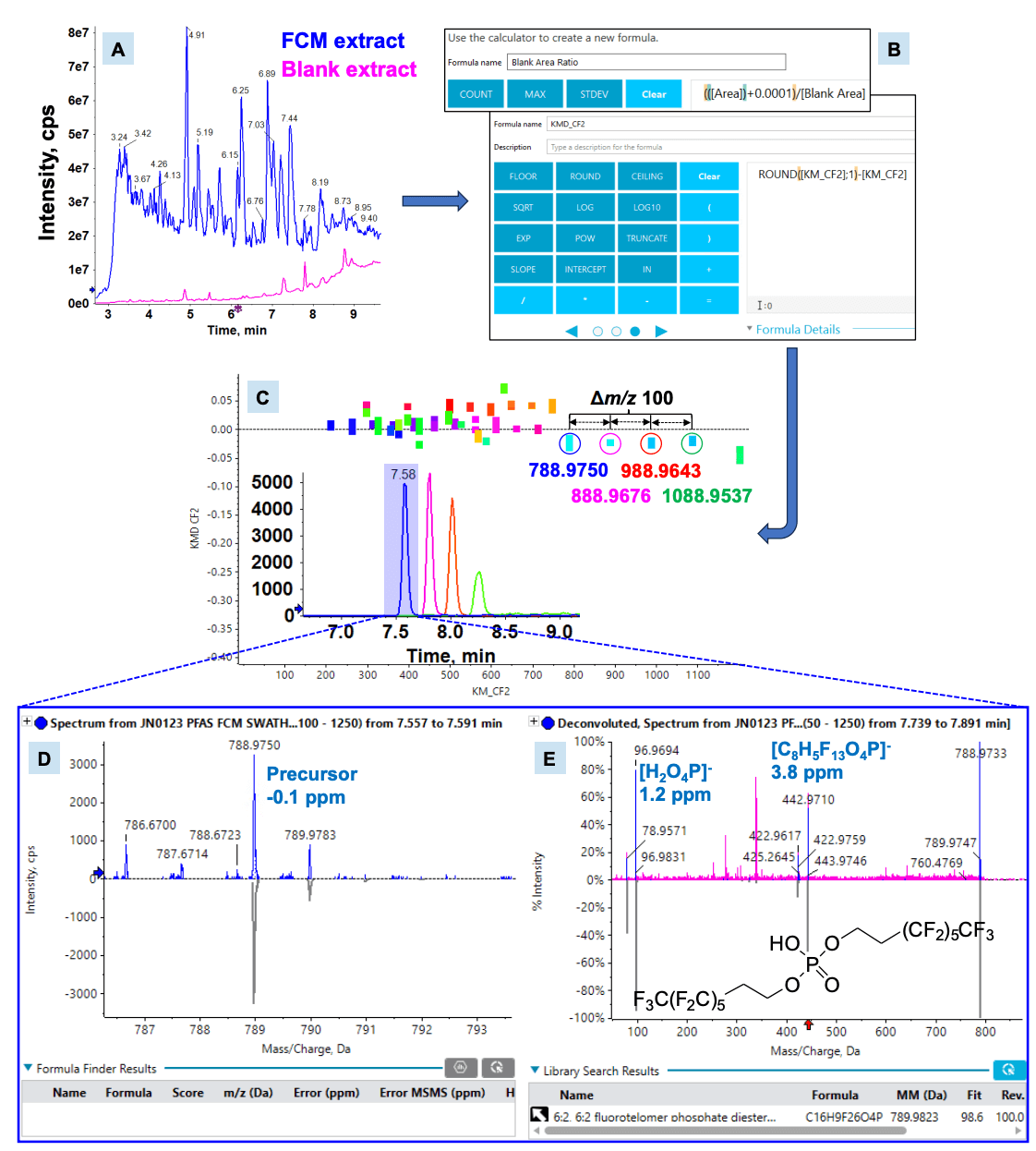 Click to enlarge
Click to enlarge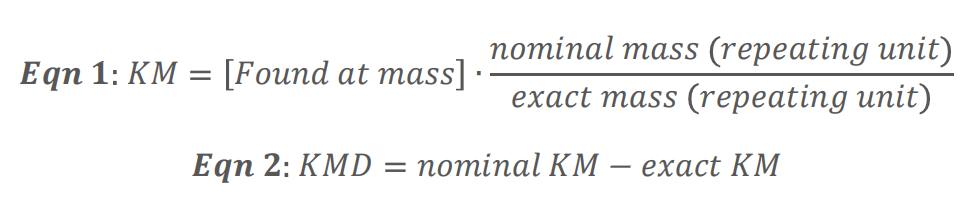 Click to enlarge
Click to enlarge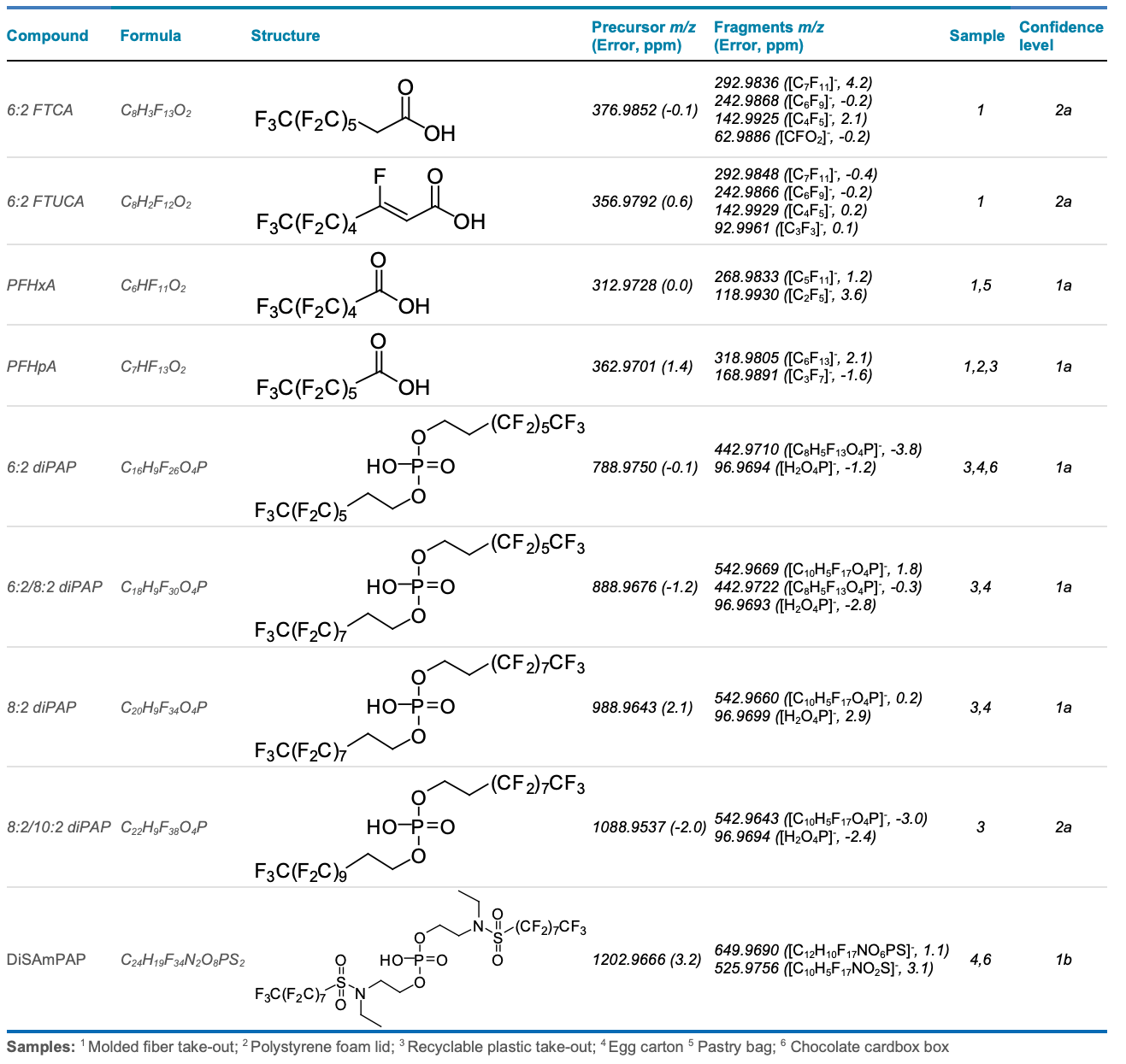 Click to enlarge
Click to enlarge