Comprehensive novel psychoactive substance (NPS) and synthetic opioids screening in dried blood spots (DBS) using high-resolution mass spectrometry
Using SWATH acquisition on the SCIEX X500R QTOF system
Marta Massano1,2, Daniele Di Corcia1, Marco Vincenti1,2, Alberto Salomone1,2 and Pierre Negri3
1Centro Regionale Antidoping e di Tossicologia “A. Bertinaria”, Orbassano, Turin, Italy; 2Dipartimento di Chimica, Università degli Studi di Torino, Turin, Italy; 3SCIEX, USA
Abstract
A comprehensive workflow is presented combining the use of the SCIEX X500R QTOF system with a fast and optimized DBS sample extraction procedure for the detection of a panel of 131 NPS including synthetic cannabinoids, synthetic cathinones and hallucinogens as well as fentanyl analogs and synthetic.

Introduction
The prevalence of novel psychoactive substances (NPS) on the recreational drug market continues to fuel the ongoing opioid crisis. NPS are designer drugs created to mimic the effects of common illicit drugs that vary greatly in potency and purity compared to prescription preparations. The emergence of new substances has been linked to a drastic increase in intoxications and accidental fatalities which pose serious publich health risks and legal challenges. As newer NPS are synthesized and introduced to the recreational drug market, timely and comprehensive analytical drug screening approaches capable of including a variety of drug types and chemistries are critically needed in the forensic laboratory.
NPS screening can be performed in a variety of biological matrices such as blood, serum, plasma or urine. In recent years, the use of dried blood spots (DBS) has become a straightforward alternative that significantly simplifies and shortens sample preparation compared to other approaches. Compared to conventional matrices, DBS analysis provides the benefits of small sample volume requirement, long term stability, minimal risk of sample degradation or adulteration as well as logistical advantages such as the absence of storage requirements.
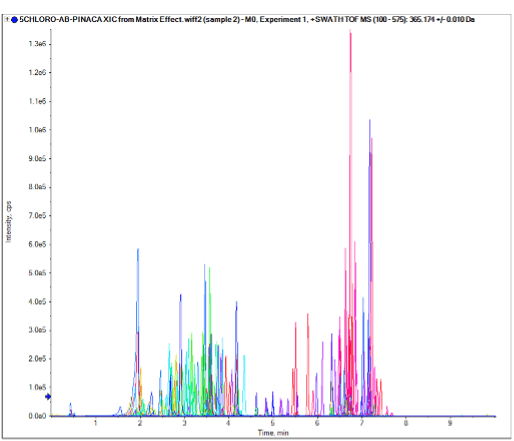
In this technical note, a comprehensive workflow is presented that uses the SCIEX X500R QTOF system in combination with a fast and optimized DBS sample extraction procedure for the detection of a panel of 132 NPS including synthetic cannabinoids, synthetic cathinones, dissociatives, hallucinogens, fentanyl analogs, synthetic opioids and some metabolites (Figure 1). This robust NPS screening workflow provides reproducible, accurate and precise quantification of low level of analytes with a wide range of physical and chemical properties.
Figure 1. Rapid detection of the 132 NPS targeted, extracted from DBS. Chromatographic profile shows the extracted ion chromatogram (XIC) resulting from the optimized LC conditions, using a spiked human whole blood calibrator solution containing the 132 NPS.
Key features of NPS screening in DBS
- Fast and optimized sample extraction procedure in combination with a robust detection method using SWATH acquisition enables detection levels ranging from 1.3 to 6.3 ng/mL for the 132 molecules included in the panel
- Method showed extraction efficiency ranging from 31% to 98% (average of 54%) and ion suppression corrected by internal standard between -69% and 10% (average of -28%) for all the NPS included in the panel
- Performance of the method resulted in excellent correlation (R2 >0.99) for all analytes included in the NPS panel
- Optimal precision (below 30% for synthetic cannabinoids and below 20% for all the other NPS classes) and accuracy (below ±20% for most NPS included in the panel) were observed
- Method can be extended to include a larger number of designer drugs as new NPS emerge onto the recreational drug market .
Experimental details
Target analytes and solutions: A total of 132 molecules and metabolites including 53 synthetic cannabinoids, 47 synthetic cathinones, dissociative and hallucinogens, 32 fentanyl analogs and synthetic opioids as well as 13 deuterated internal standards were purchased from Cerilliant Corporation (Round Rock, TX). Two solutions were prepared in water: a standard solution containing the 132 NPS and an internal standard solution containing the 13 deuterated standards. Table 1 lists the analyte class and name, LOD and LOQ, matrix effect (ion suppression), extraction efficiency (recovery %), linear correlation value (R2) as well as the inter- and intra-day precision and accuracy for each of the 132 NPS included in the panel.
Calibrator preparation: Five levels of calibrators were prepared by spiking the standard solution containing the 132 target analytes in human whole blood to final concentrations ranging from 5 to 100 ng/mL.
Sample preparation and DBS sample extraction procedures: Protein saver cards (also known as DBS cards) were purchased from Whatman (Piscataway, NJ). Human whole blood calibrator samples spiked with various concentrations of the 132 molecules were spotted onto the DBS cards. NPS were extracted using the sample extraction procedure summarized in Figure 2.
Figure 2. NPS extraction workflow from DBS cards. A 10-step sample extraction protocol was optimized to selectively extract the 132 NPS from DBS cards for analysis using the SCIEX X500R system.
Liquid chromatography: UHPLC separation was performed using the SCIEX ExionLC AC system and a Phenomenex C18 column (100 x 2.1 mm, 1.7 µm, 00D-4475-AN) held at 45ºC. Mobile phases used consisted of water, acetonitrile and modifiers. The LC flow rate was 0.5 mL/min and the total run time was 10 min. The injection volume was 5 µL.
Mass spectrometry: MS and MS/MS data were collected for each sample using SWATH acquisition on the SCIEX X500R QTOF system in positive ionization mode. Data acquisition was TOF MS scan followed by 18 MS/MS scans using variably sized Q1 windows covering a mass range from 100 to 575 Da. Data was acquired using SCIEX OS software 1.5.
Data analysis: Data processing was performed using SCIEX OS software 1.5.
Developing a robust workflow for accurate mass detection of NPS extracted from DBS
Blank human whole blood samples were spiked with each of the prepared calibrators. A volume of 30 µL of each calibrator solution was spotted onto a DBS card. The NPS were extracted from the DBS using the aforementioned procedure and injected in triplicate over the course of two consecutive days to build a data processing method. Figure 1 shows the chromatographic profile of the 132 molecules resulting from the optimized LC conditions, using a human whole blood calibrator solution spiked at 100 ng/mL with the standard solution and prepared as discussed previously.
Optimized sample preparation procedure leads to high extraction efficiency and low ion suppression
Developing a sample preparation method capable of selectively extracting various classes of NPS with a wide range of physical and chemical properties, such as those included in this panel, is essential to attaining reliable results. To that end, the efficiency of the sample extraction procedure used in this workflow was investigated by calculating the extraction efficiency (measured as % recovery using the 75 ng/mL calibrator solution) and ion suppression (measured as mean matrix effect using the 10 ng/mL calibrator solution) for the 132 molecules included in this panel. These two sample preparation key indicators affect analyte quantification, assay linearity and the precision and accuracy of the method. The recovery values were calculated as the ratio between the average (n=2) peak areas of each analyte spiked before and after the extraction procedure, as a percentage. The ion suppression was calculated as the ratio between the average (n=2) peak areas of each analyte in neat solvent and in post-extraction spiked matrix as a percentage. The recovery values ranged between 31 and 98% (average of 54%) and the ion suppression compensated by internal standard ranged between -69% and 10% (average of -28%), proving the efficacy of the sample extraction method used in this study.
SWATH acquisition leads to precise and accurate NPS quantification
Reliable quantification of drugs extracted from DBS is critical to ensure the robustness of a developed method. In this study, SWATH acquisition was used as the detection method to generate comprehensive and high-quality MS/MS spectra of every detectable NPS extracted from the DBS cards. This acquisition method is particularly useful for NPS screening and identification as each acquisition file contains a digital record of the sample analyzed, enabling re-interrogation of a pre-acquired sample data set to search for the presence of an NPS that was not known or targeted at the time of sample analysis.The series of five calibrator solutions ranging from 5 to 100 ng/mL were injected to evaluate the quantitative performance of SWATH acquisition and its ability to accurately measure various levels of NPS extracted from DBS with a high level of linearity, precision and accuracy.
Figure 3 shows representative extracted ion chromatograms (XICs) for each of the drugs classes included in this panel. Figure 3 shows the XIC traces for A) JWH-007, a synthetic cannabinoid, B) butylone, a stimulant and C) acetyl fentanyl, a fentanyl analog, at each of the five concentrations across the calibration range (5 to 100 ng/mL). Limits of quantification (LOQs) ranged from 6 ng/mL for 1-(5-fluoropentyl)-N-(2-phenylpropan-2-yl)-1H-indazole-3-carboxamide (5F-CUMYL PINACA) and 3,4-dicloro-N-[(1-(dimetilammino)cicloesil)metil] benzamide (AH-7921) up to 15 ng/mL for 2-(2,5-dimethoxy-4-propylphenyl)ethan-1-amine (2C-B).
Figure 3. Representative extracted ion chromatograms (XICs) for each of the drug classes included in the panel. XIC traces for A) JWH-007 (a synthetic cannabinoid), B) butylone (a stimulant) and C) acetayl fentanyl (a fentanyl analog) from the series of five calibrator solutions ranging from 5 to 100 ng/mL.
Using a non-targeted detection method that consistently delivers reproducible and accurate results for every injection of every batch is critical to the overall performance of the assay. In this experiment, a series of injections were performed over the course of two consecutive days to evaluate the reproducibility and robustness of the method. Calculated concentration accuracies (%CV) were found to be within 80% and 120% for most NPS, precision (%bias) below 30% for synthetic cannabinoids and below 20% for all other NPS classes. Overall, the assay showed great reproducibility over the course of two consecutive days and across the five calibrator solutions, proving the quantitative robustness of the overall workflow.
Calibration curves
Calibration curves were generated for each of the 132 molecules in the panel. Figure 4 shows the resulting regression lines for A) the 53 synthetic cannabinoids, B) the 47 synthetic cathinones, dissociative and hallucinogens and C) the 32 fentanyl analogs and synthetic opioids. The calibration curves demonstrated excellent linearity with R2 greater than 0.99 for all the NPS targeted in the panel. Overall, the assay showed excellent reproducibility, precision, accuracy and linearity, proving the robustness of the extraction procedure and quantitative performance of the detection method used for this workflow. Table 1 summarizes the statistical results for the 132 molecules targeted in this workflow and includes the analytes’ class and name, LOD and LOQ, extraction recoveries values, matrix effect mean, as well as the inter- and intra-day precision and accuracy.
Figure 4. Excellent linearity for the 132 molecules included in the panel. Calibration curves resulting from the calibration series for A) 53 synthetic cannabinoids, B) 47 synthetic cathinones, dissociative and hallucinogens, and C) 32 fentanyl analogs and synthetic opioids. Excellent linear response and sensitivity were observed with R2 values greater than 0.99 for all the molecules included in this panel.
Conclusions
A comprehensive workflow for the detection of a panel of 132 NPS extracted from DBS was successfully developed using the SCIEX X500R QTOF system. A rapid and optimized sample extraction procedure in combination with a highly selective MS/MS method using SWATH acquisition enabled robust and reproducible detection of NPS with a wide range of physical and chemical properties. The use of SWATH acquisition enabled generation of a comprehensive digital archive of each DBS sample via acquisition of high-quality and sensitive MS/MS spectra for every detectable NPS present in the sample.
The method showed analyte recoveries averaging 54% and ion suppression averaging -28% for all the molecules. High reproducibility was observed over the course of two consecutive days, with accuracy (%CV) within 20% of 100% for most NPS, precision (% bias) below 30% for synthetic cannabinoids and below 20% for all other NPS classes. In addition, the performance of the method resulted in excellent correlation (R2 >0.99) for all analytes included in the NPS panel across the calibration range (5 to 100 ng/mL). Overall, the method showed excellent quantitative results, with quantification limits ranging from 4 to 10 ng/mL for the analytes included in the panel. In addition, this method can be used to create a digital archive of NPS present in a blood case sample at the time of sample collection. The results also suggest that this robust method can be expanded to include new NPS emerging from the recreational market and used as robust workflow for accurate and precise NPS quantification.
Table 1. Statistical results for the 132 molecules targeted in this workflow. The table includes the compound class and name, LLOD and LLOQ, recovery values at 75 ng/mL, matrix effect mean at 10 ng/mL, as well as the inter-day and intra-day precision (%CV) and accuracy (range bias%) for the 131 NPS included in this study.
Table 1. Statistical results for the 132 molecules targeted in this workflow. Continued |
Table 1. Statistical results for the 132 molecules targeted in this workflow. Continued
Table 1. Statistical results for the 132 molecules targeted in this workflow. Continued
 Click to enlarge
Click to enlarge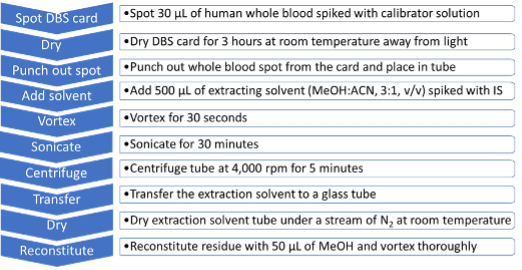 Click to enlarge
Click to enlarge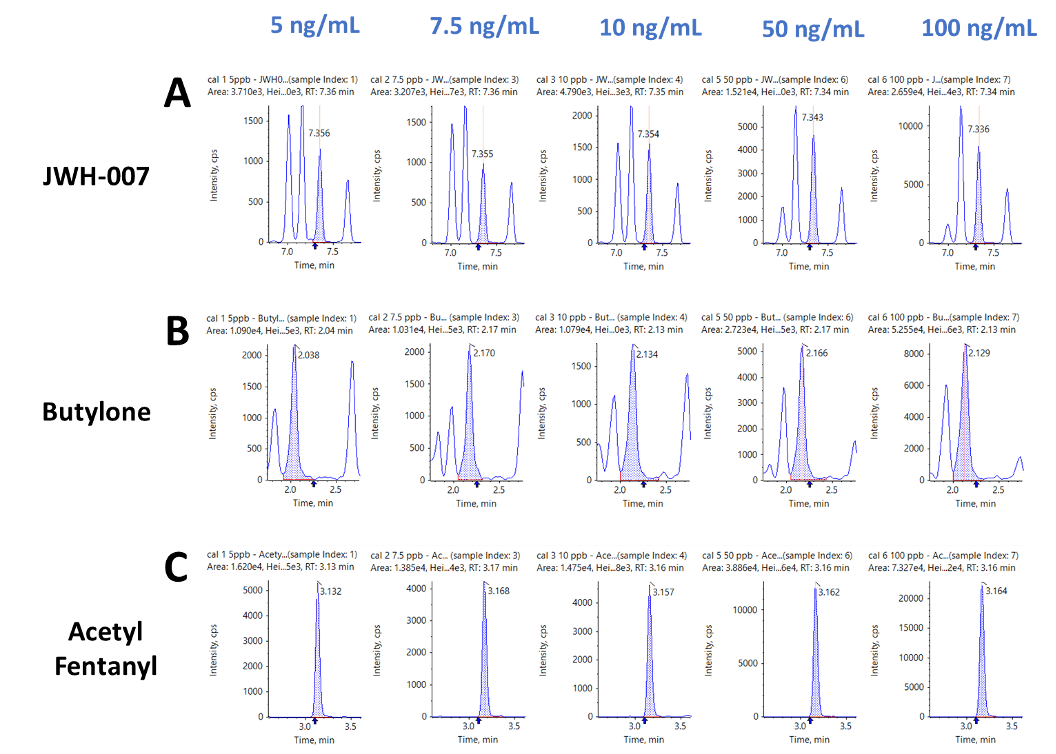 Click to enlarge
Click to enlarge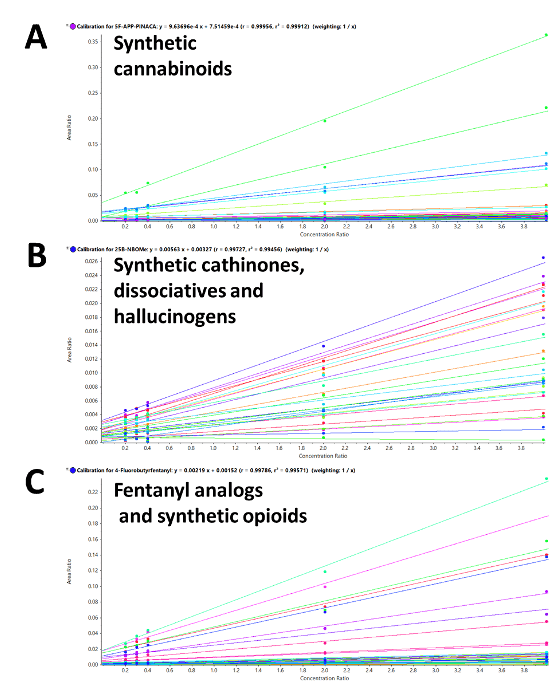 Click to enlarge
Click to enlarge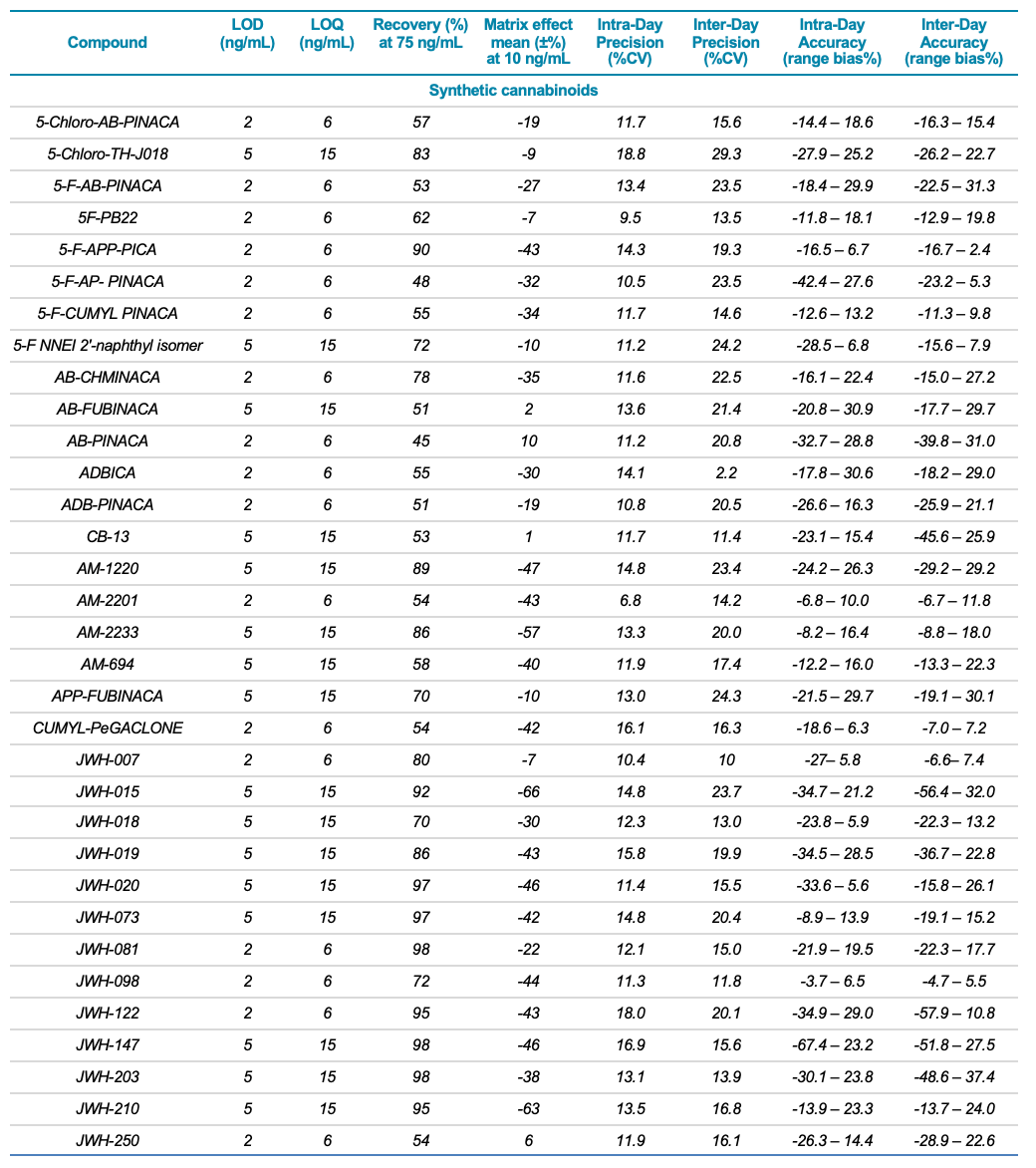 Click to enlarge
Click to enlarge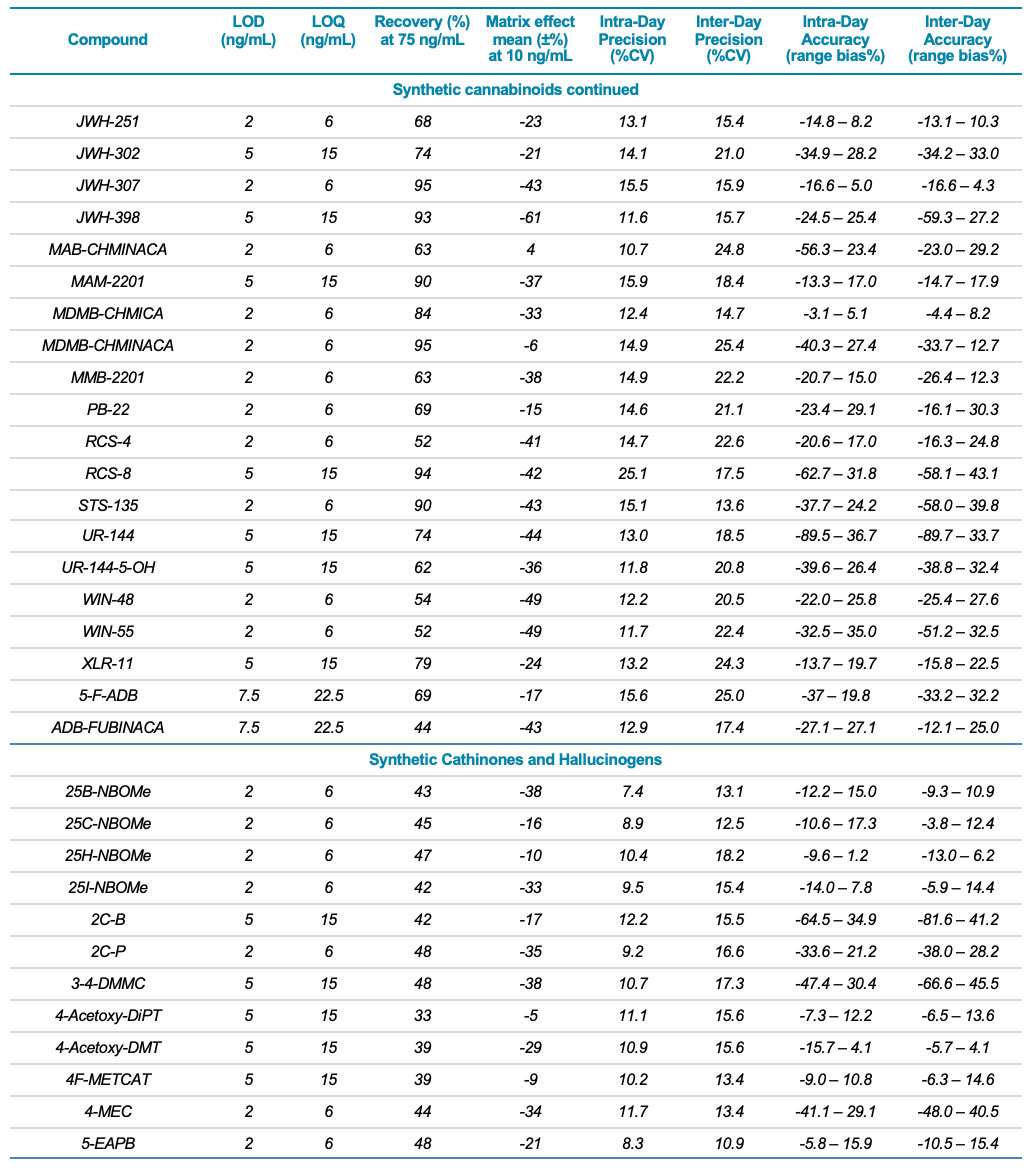 Click to enlarge
Click to enlarge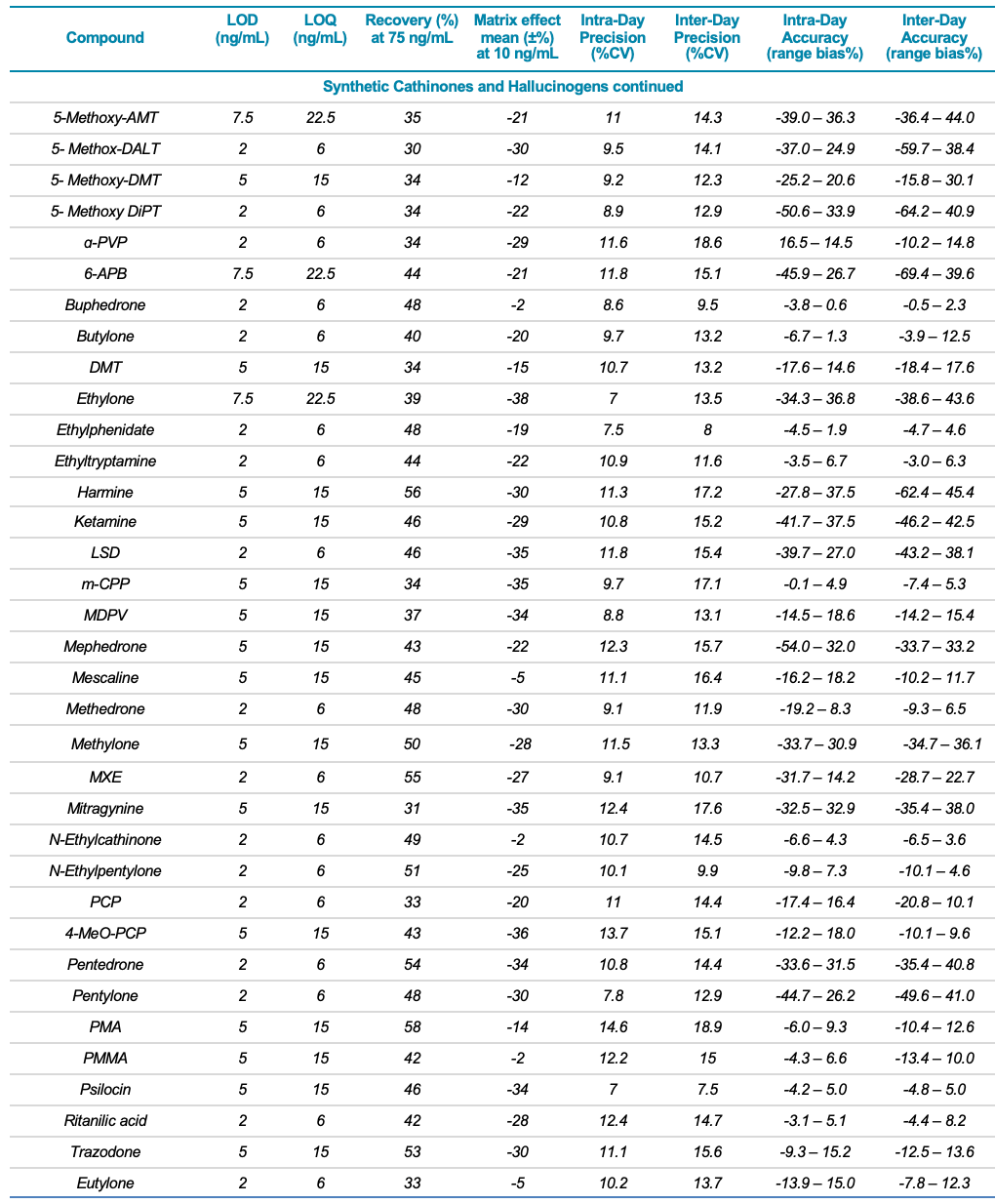 Click to enlarge
Click to enlarge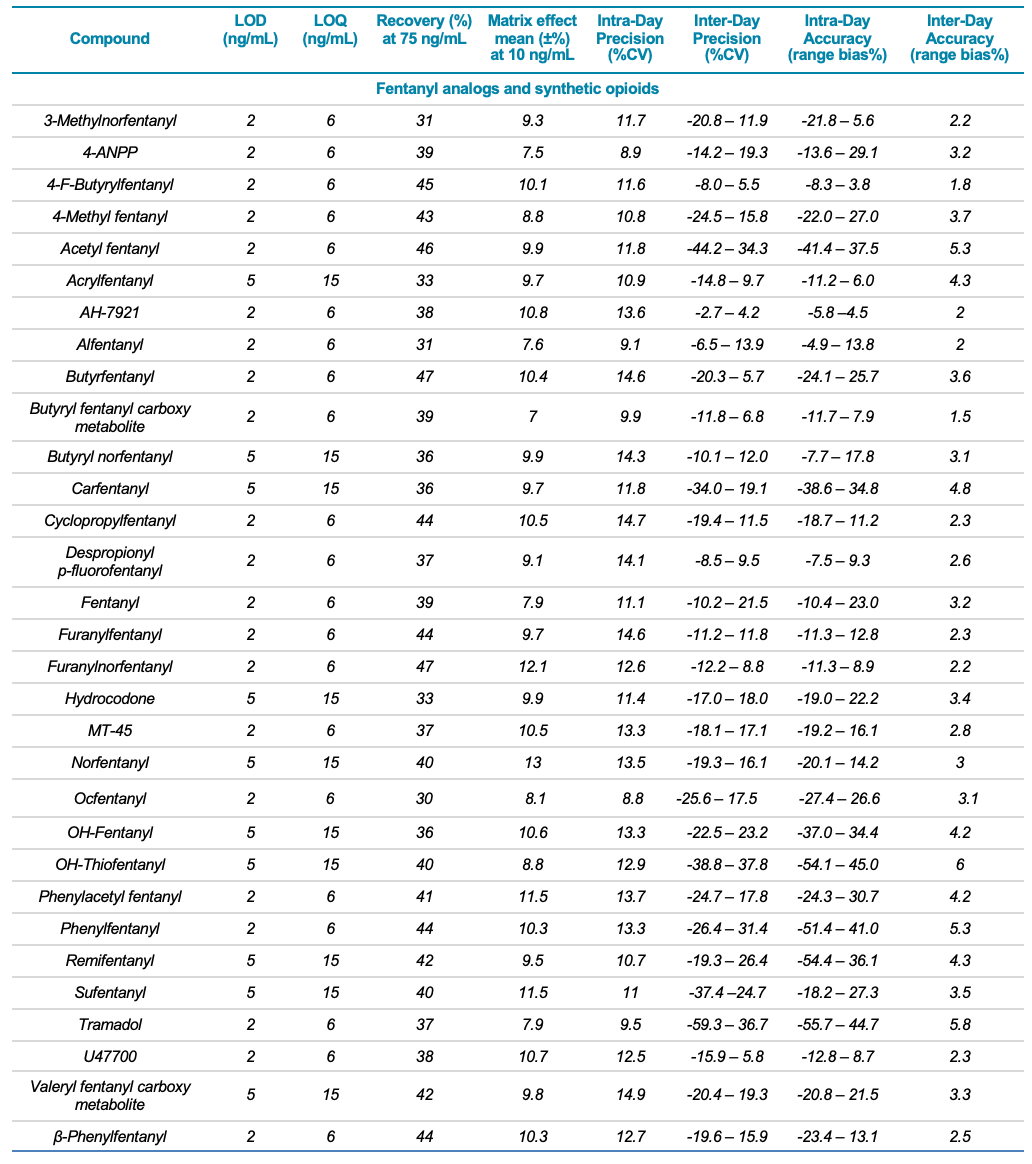 Click to enlarge
Click to enlarge