Rapid identification and quantification of novel psychoactive substances in human whole blood using SWATH® Acquisition
Using data independent acquisition on the SCIEX TripleTOF®5600+ LC-MS/MS System
Pierre Negri1 and Alex J. Krotulski2,3
1SCIEX, USA; 2Temple University, USA, 3Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, USA
Abstract
A sensitive and comprehensive quantitative drug screening workflow for 30 NPS representatives of the drug classes listed in the DEA’s Emerging Threat Report is described. Workflow was streamlined using a liquid-liquid extraction combined with SWATH® Acquisition on the TripleTOF® 5600+ System. This targeted screening method enabled rapid and confident identification of multiple classes of NPS drugs in human whole blood.

Introduction
Every year, the Drug Enforcement Administration (DEA) Special Testing and Research Laboratory publishes a yearly Emerging Threat Report listing the Novel Psychoactive Substances (NPS) the agency has seized and analyzed.1 This concise report is meant to provide an annual snapshot of current and emerging NPS markets in the United States. As the surge of novel synthetic opioids and other synthetic drug classes continue to pose serious public health and safety problems, timely and comprehensive drug screening approaches are critically needed in the forensic laboratory to quickly and accurately identify these emerging novel substances.
The combination of LC separation coupled to tandem mass spectrometry detection (e.g., LC-MS/MS, LC-QTOF-MS) provides forensic investigators the speed and confidence required to reliably identify novel drugs of abuse and other toxicology compounds present in a variety of complex matrices. In addition, acquisition of accurate mass data and analyte-specific MS/MS fragment spectra provides increased confidence in compound identification. More specifically, high-resolution mass spectrometry (HRMS) offers forensic laboratories a powerful tool for the detection and identification of NPS by reliably obtaining comprehensive MS/MS spectral fragment information on every detectable component in the sample at low analyte concentration with high levels of selectivity and sensitivity.
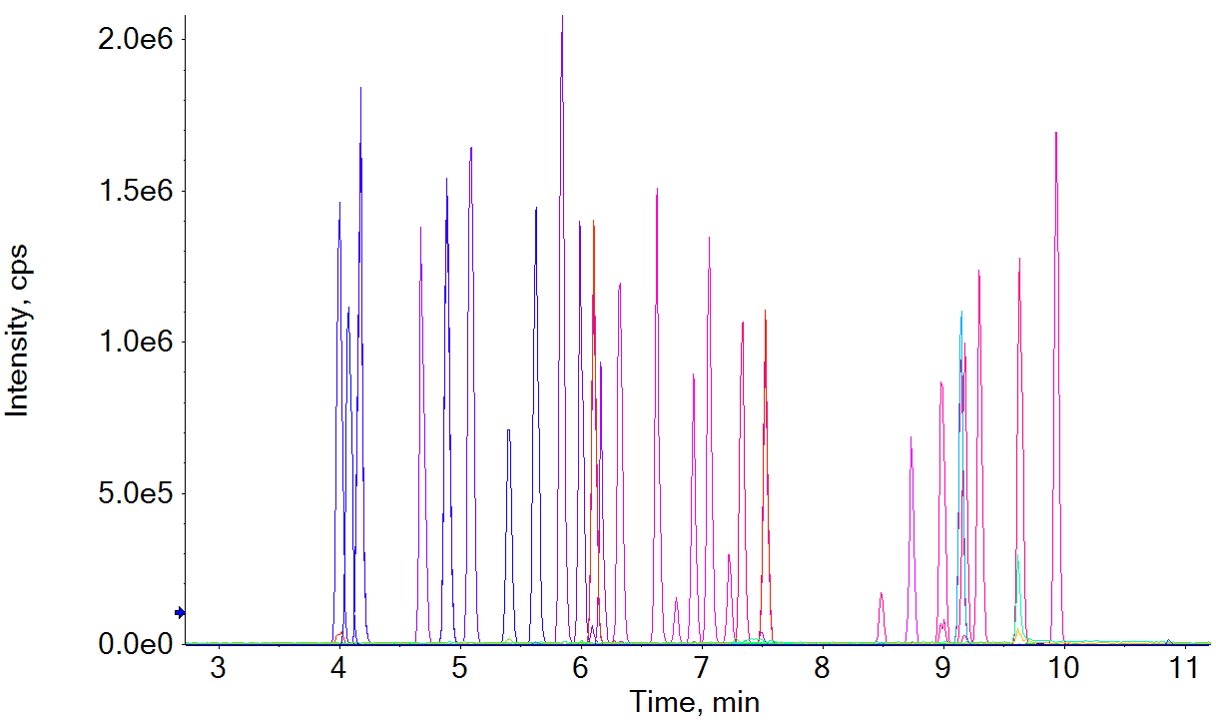
Figure 1: Chromatographic profile of the NPS panel by LC-MS analysis. Extracted Ion Chromatograms (XICs) resulting from total or near baseline separation of 42 compounds in a 15-minute runtime.
Key features of SWATH Acquisition for NPS identification and quantitation
- The NPS quantitation panel consisted of 30 representative NPS listed in the DEA’s Emerging Threat Report, as well as 12 internal standards. The NPS qualitative panel consisted of more than 600 NPS and NPS metabolites
- The high scanning speed (up to 100 Hz for single collision energy) of the TripleTOF 5600+ system allowed detection of all target analytes in the NPS panel
- SWATH Acquisition generated comprehensive and high-quality MS/MS spectra with no loss in sensitivity, enabling confident drug identification using spectral library searching
- Analyte extraction recoveries were demonstrated to be greater than 80%, allowing sub ng/mL detection limits of these drugs in a complex biological matrix while maintaining correlation and precision for all compounds across the calibration range
- The TripleTOF 5600+ System enabled simultaneous quantitation and confirmation of the NPS by utilizing the more selective MS/MS information as well as using both ion ratio and MS/MS library searching for confident identification of all the NPS used in this workflow
Figure 2: MS/MSALL with SWATH Acquisition for screening of Novel Psychoactive Substances (NPS). In SWATH Acquisition, Q1 isolation windows are stepped across the mass range in an LC timescale, transmitting populations of analytes for fragmentation, and high-resolution composite MS/MS spectra are acquired at each step. This produces a richer MS/MS spectrum which is a composite of all the analytes within that Q1 m/z window. MS/MS acquisition is performed across the full m/z range of target analytes, ensuring MS/MS fragment data is collected on every detectable compound. Because the fragment ions are generated using high-resolution acquisition, detected compounds can be accurately identified through extraction of the specific, accurate mass product ions (in addition to extract of precursor ions from the TOF-MS scan).
Experimental details
Sample Preparation: A total of 30 target analytes were selected for quantitation representing all of the drug classes reported in the DEA’s Emerging Threat Report, in addition to the >600 NPS incorporated into the screening workflow. These NPS consisted of 7 synthetic cannabinoids, 2 dissociatives, 3 hallucinogens, 6 stimulants, 5 benzodiazepines and 7 opioids. A full list of the 30 NPS used in this method is detailed in Table 1, including accurate mass information and fragment ion masses used for ion ratio determinations. Extraction of whole human blood samples was performed according to the liquid-liquid extraction (LLE) procedure shown in Figure 3.
Figure 3. Liquid-Liquid Extraction (LLE) workflow for human whole blood. A 10-step extraction protocol was used for selectively extracting the 30 target analytes and internal standards from human whole blood samples for analysis with the TripleTOF 5600+ System.
Internal standards: Methylone-D3, Alpha-PVP-D8, Ketamine-D4, PCP-D5, Diazepam-D5, Alprazolam-D5, U-47700-D6, Fentanyl-D5, JWH-018-D9, Cyclopropyl Fentanyl-D5, AM-2201-D5, and AB-FUBINACA-D4 were used as internal standards.
Calibrator preparation: A 10 ng/µL stock standard solution mixture containing all the NPS used for quantitation in this study was prepared via dilution of 1 mg/mL stock standards with methanol. A series of calibration solutions were prepared using the 10 ng/µL stock standard solution mixture to evaluate the dynamic range. These, in turn, were used to spike 500 µL of human whole blood in order to prepare a calibration series covering concentrations ranging from 1 ng/mL to 100 ng/mL. Finally, 25 µL of the 1 ng/µL IS spiking stock solution was added to each human whole blood mixture.
Liquid chromatography: UHPLC separation was performed on a Phenomenex Kinetex C18 column (50 × 3 mm, 2.6µm, 00B-4462-Y0) on the Shimadzu Nexera system. Mobile phase A (MPA) and mobile phase B (MPB) were ammonium formate with formic acid and formic acid in methanol and acetonitrile, respectively. The injection volume was 10 µL and the total LC runtime was 15.5 minutes.
Mass spectrometry: MS and MS/MS data were collected using SWATH Acquisition on the SCIEX TripleTOF 5600+ System with Analyst 1.7 Software. Each SWATH Acquisition scan began with a TOF-MS experiment (50-510 Da).
Optimized Liquid-Liquid Extraction (LLE) procedure leads to high NPS recovery
One of the main challenges associated with the detection of analytes extracted from a biological matrix, such as whole blood, is the presence of complex matrix contents, some of which are structurally similar to the analytes in this panel. As a result, robust and reliable extraction procedures are critical in achieving the desired reproducibility, linear response, and limits of quantitation (LOQ). To assess the efficiency of the LLE procedure used in this experiment, the recovery (RE) was calculated using 80 ng/mL of each NPS. The average (n=3) peak area ratio (analyte area to internal standard area) of a pre-spike sample can be depicted as “C” and the peak area ratio of a post-spike sample as “B”; therefore, the RE value for each analyte can be calculated as follows:
RE (%) = C/B x 100 (1)
The LLE procedure used in this experiment demonstrated excellent recoveries of the analytes used in this panel (>80%). The RE values for each NPS are summarized in Table 1. The high recovery values for the analytes in the panel allowed reliable quantification, which is only possible through the implementation of an optimized, universal LLE procedure.
Analytical sensitivity of the TripleTOF 5600+ LC-MS/MS System
Following the LLE procedure, 10 µL of reconstituted solution were injected for each calibrator sample. A processing method for the 30 target analytes and 12 internal standards was developed to review the SWATH Acquisition data in SCIEX OS Software 1.5. Detection and integration of the peaks was easily accomplished using the MQ4 algorithm in Analytics, which facilitated selection of the correct peak within the viewing window of closely eluting isobaric analytes. As this data was acquired using SWATH Acquisition, peak area ratio (PAR) and ion ratio (IR) determinations can be performed by extraction of accurate mass fragment ions the MS/MS spectra. Quantitation was achieved by plotting the PAR of the analyte to internal standard. The IR was calculated by dividing the area of the two most intense fragments, the quantifier ion and qualifier ion.
Figure 4 shows the extracted ion chromatogram (XIC) traces for both the quantifier (428.0717 Da à 121.0650 Da) and qualifier (428.0717 Da à 91.0544 Da) ions for 25I-NBOMe. The XIC traces for all the compounds showed a high level of consistency and precision across the calibration series. Quantitation results were processed using SCIEX OS Software, which allowed fast data processing with less manual intervention and streamlined reporting tools.
Figure 4: Streamlined data processing of 25I-NBOMe using SCIEX OS 1.5 Software. XIC traces and peak integration of 25I-NBOMe using the quantifier (428.0717 Da à 121.0650 Da) and qualifier (428.0717 Da à 91.0544 Da) ions with the ion ratio line overlay displayed. The XIC traces demonstrate reliable quantitation from 1 to 100 ng/mL.
Calibration curves were generated to evaluate the response, dynamic range and quantification performance (precision and accuracy) of the assay. Figure 5 shows the results from the calibration series performed for 25I-NBOMe including the calibration curves for both ions and statistics of quantitation generated using this comprehensive method. Seven levels of calibrators ranging from 1 to 100 ng/mL were used to determine the ion ratio criteria for the quantifier and qualifier ions of 25I-NBOMe. The results demonstrated excellent correlation of the generated regression curves covering a broad dynamic range from 2 to 3 orders of magnitude. The assay showed excellent precision (<15%) and accuracy (>95%), and the R2 values for the quantifier and qualifier ions were 0.99965 and 0.99967, respectively. Full quantification was achieved with SCIEX OS 1.5, designed for quick, intuitive and streamlined data processing with accurate and reliable results.
Figure 5: Accurate and reliable quantitation using SCIEX OS Software. Calibrations curves resulting from the calibration series for 25I-NBOMe from 1 to 100 ng/mL for both the quantifier and qualifier ions. The calibration curves show excellent correlation, precision and accuracy.
Table 1. List of analytes (including chemical formula, precursor and fragment accurate mass information and fragment ions used for ion ratio determination, retention time, and internal standard) and recovery values for each of the 32 NPS in this workflow.
Full scan MS/MS with SWATH Acquisition leads to accurate compound identification
SWATH Acquisition is a non-targeted method that provides a comprehensive qualitative and quantitative analysis of complex samples by acquiring full scan high resolution MS and MS/MS data on all detectable compounds eluting during an LC run. It provides selective MS/MS detection of compounds extracted from biological samples where matrix interference is common.
A great advantage to the use of the SCIEX OS Software is that it allows for simultaneously quantification using ion ratio determination and library searching confirmation. This processing is performed at the same time and allows for the results to be displayed within one window. Figure 6 (Bottom) shows the peak integration for an extracted ion chromatogram (XIC) of valeryfentanyl, as well as the TOF MS and TOF MS/MS spectra with library search match. A library fit score of 100 for the identification of valerylfentanyl provides excellent confidence for a definitive detection of this NPS. Also displayed in Figure 6 (Top) are the results from the calibration curves resulting from the peak area integration of the compound at concentrations ranging from 1 to 100 ng/mL. Overall, excellent linearity and reproducibility was observed across the entire concentration range assessed for all the compounds in this panel. Using this comprehensive method, the 30 NPS used in this study were quantitatively identified and confirmation was achieved using MS/MS library searching with excellent library scores (>80% for all NPS analytes).
The processing method used in this workflow was optimized on the SCIEX TripleTOF 5600+ System. However, it is worth noting that the same processing method can be easily transferred to the SCIEX X500R QTOF System and used for additional screening. This feature allows data sets from other panels to be re-processed when newly identified forensic targets are discovered.
Figure 6. Comprehensive and streamlined data processing using SCIEX OS Software leads to accurate compound identification. Using multiple acceptance criteria enabled the accurate identification of all NPS showcased in this quantitative workflow. (Top) Calibration series for valerylfentanyl ranging from 1 to 100 ng/mL for both the quantifier and qualifier ions passing the acceptance criteria (green traffic light) for ion ratio, mass error, isotope and library match. Also included are the mass error and the library score for valerylfentanyl (Bottom), including the XIC, TOF-MS spectrum and MS/MS spectrum along with spectral library match.
Conclusions
A comprehensive drug screening workflow using the TripleTOF 5600+ System for the analysis of NPS listed in the DEA’s Emerging Threat Report is described. The results demonstrate the use of SWATH Acquisition in combination with SCIEX OS Software makes this workflow easily adaptable into a forensic toxicology laboratory and compatible with high throughput laboratory screening workflows.
- A targeted drug screening method using SWATH Acquisition was developed for fast, selective, and quantitative detection of low concentration NPS in a single analysis
- A 10-step liquid-liquid extraction protocol was rapidly implemented to extract the analytes from human whole blood
- Analyte extraction recoveries were demonstrated to be greater than 80%, enabling the analytical workflow to obtain sub ng/mL lower limits of quantification (LLOQ) for most analytes in a complex biological matrix
- The data-independent acquisition strategy allowed ion ratio determinations by extraction of multiple accurate mass fragment ions from the MS/MS spectra in combination with library searching for confident identification
- The workflow showed excellent precision (<15%) and accuracy (>95%), with excellent linearity resulting in R2 values above 0.990 for all analytes
References
- Emerging Threat Report, DEA, 2017.
 Click to enlarge
Click to enlarge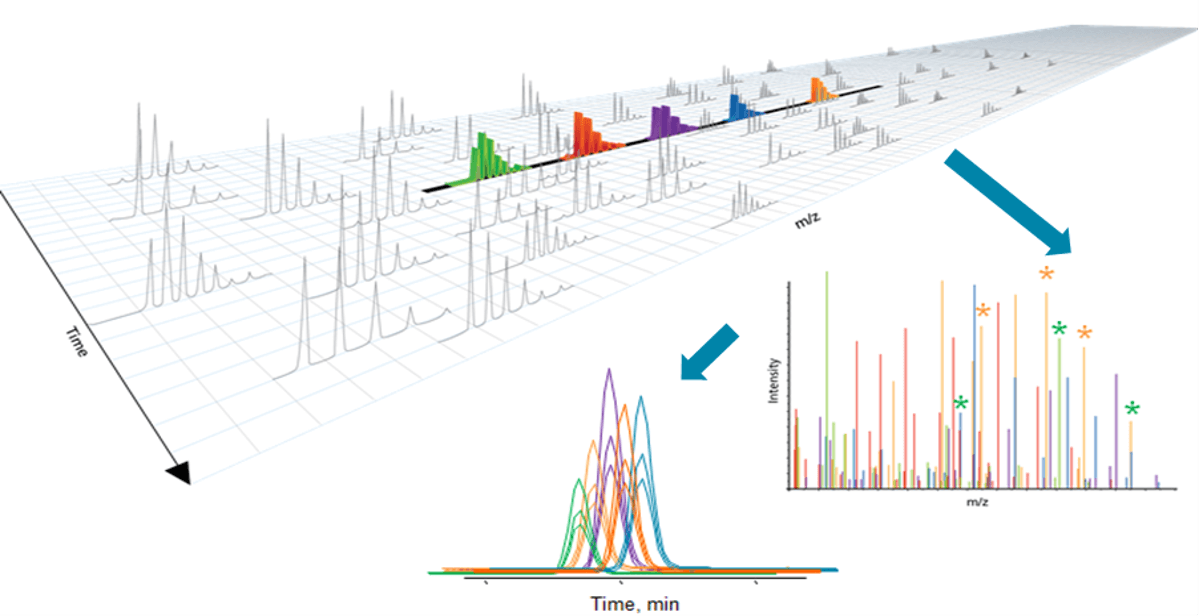 Click to enlarge
Click to enlarge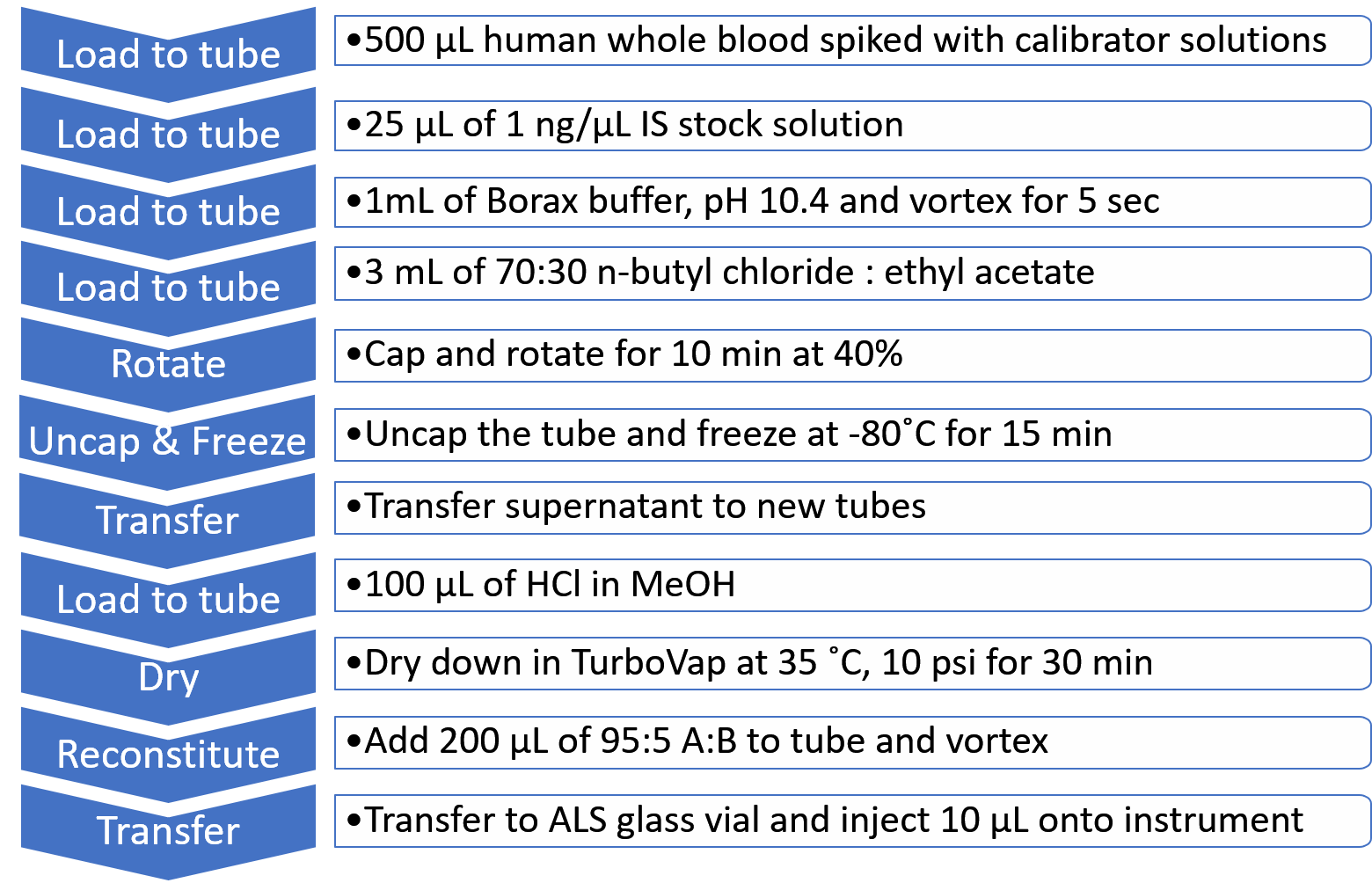 Click to enlarge
Click to enlarge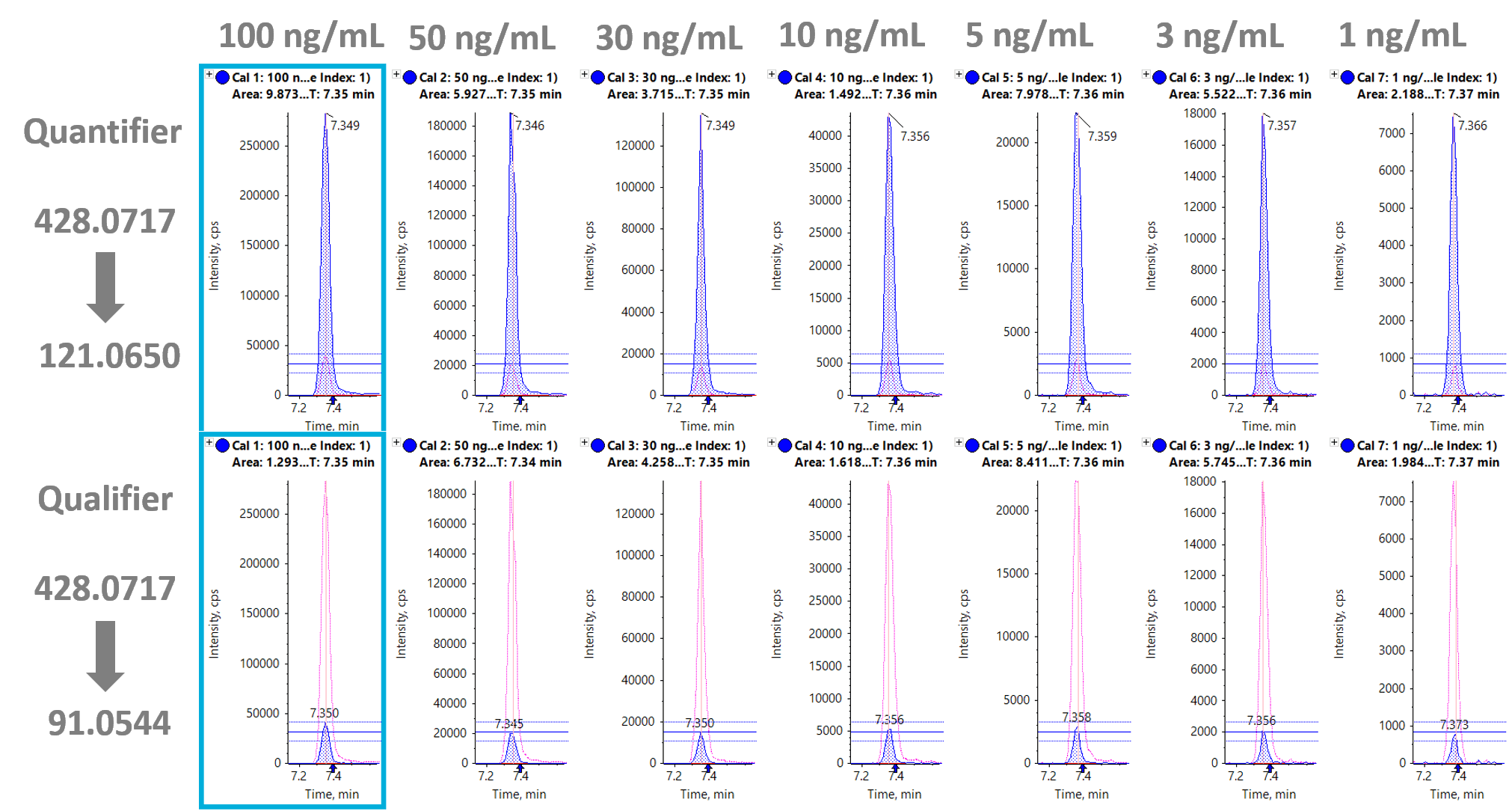 Click to enlarge
Click to enlarge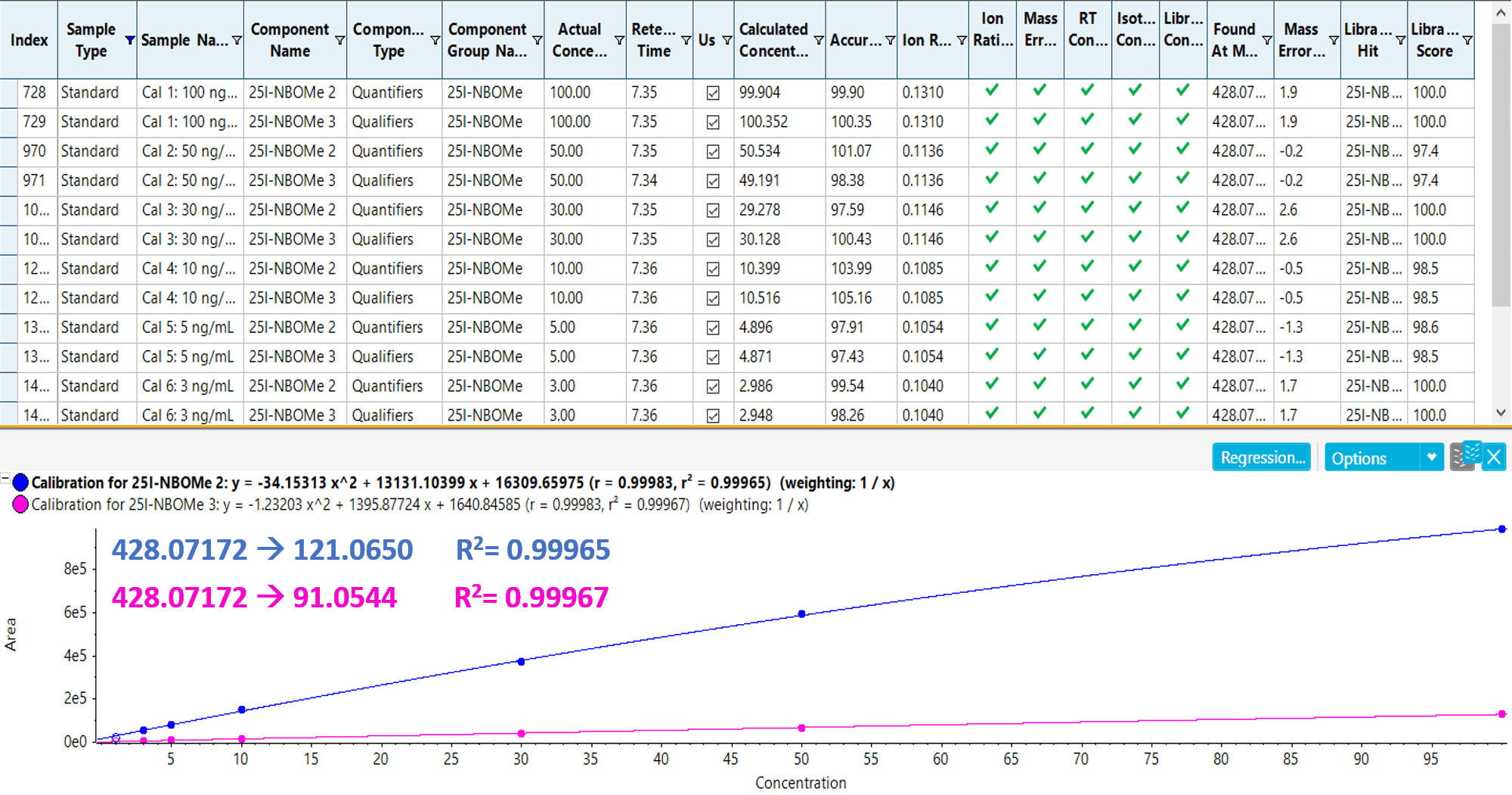 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge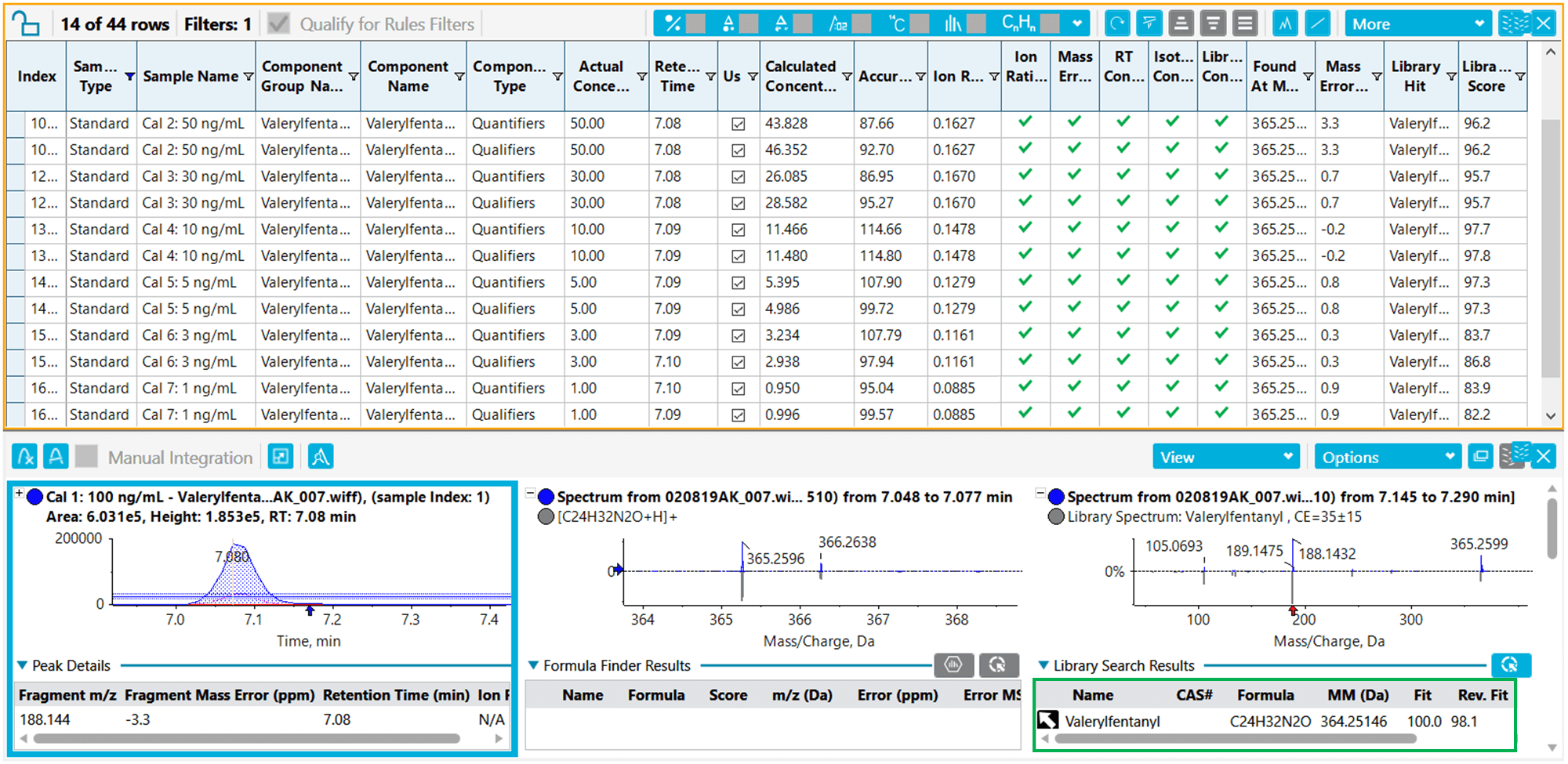 Click to enlarge
Click to enlarge