Accelerating synthetic biology with very fast screening of metabolites in fermentation broth
Using the Echo® MS system
Junmiao Chen, Dandan Si and Zhimin Long
SCIEX, China
Abstract
Optimization of the synthetic pathways necessary to produce a target chemical requires the ability to rapidly monitor key analytes along the pathway in real time. In the synthetic biology process this is often done in fermentation broth. Here, a method was developed using the Echo MS system to quantify 10 different metabolites out of fermentation broth. Acquisition on Echo MS system is extremely fast, the entire experiment once the assay was developed took 20 minutes to complete providing near real-time information. The same experiment using a more traditional approach with a 5 min LC-MS/MS approach would take 32 hours.

Introduction
Synthetic biology is a field emerging from the intersection of biology, engineering, chemistry and bioinformatics. One of the many important applications of synthetic biology is to reduce reliance on petrochemical refining and plant extraction, for example, by using microbial fermentation for the production of bulk chemicals and natural products.
During the process of microbial fermentation, the synthetic pathways necessary to produce the target chemical may not exist in a single organism and is usually less efficient after it is created. Therefore, it is necessary to monitor and optimize the synthetic pathway in real time to improve its efficiency.1 During real-time monitoring, optimization and post-industrial production, it is necessary to detect the content of multiple target products in the fermentation broth. Current options for rapid and high-throughput analysis of the fermentation broth pose a bottleneck in the control of the microbial fermentation production process, due to the large number of samples and the need for complex sample pre-processing. For example, a 5-minute LC method would require more than 32 hours to analyze 384 samples, whereas the Echo MS system requires only 20 minutes to run 1 method on the same 384 samples.
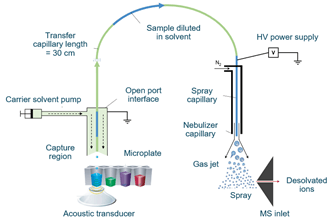
The Echo MS system uses Acoustic Ejection Mass Spectrometry (AEMS) to introduce the sample into an electrospray ionization mass spectrometry-based system for detection. The Echo MS system uses Acoustic Droplet Ejection (ADE) to precisely control the transfer of samples from the sample plate to the Open Port Interface (OPI), which is directly connected to the OptiFlow ion source on the SCIEX Triple Quad 6500+ system (Figure 1).2 This non-contact sampling method ensures that there is no residue in the sampling process and can analyze 1 sample per second, even achieving ultra-fast acquisition speeds of 3 samples per second.
Here, a method is described using the Echo MS system to quickly analyze various components in the fermentation broth, including long-chain dicarboxylic acid compounds such as succinic acid and sebacic acid. The method includes simple sample preparation and small sample amounts, and is suitable for rapid and high-throughput screening of complex samples in microbial fermentation production.
Figure 1. Acoustic Ejection Mass Spectrometry (AEMS). Samples are ejected using Acoustic Droplet Ejection (ADE) into the Open Port Interface (left) then carried through the capillary to the ESI probe (right) at the mass spectrometer for fast analysis.
Key features of the Echo MS system for high-throughput quantification
- Rapid analysis speed of 1 second per sample permits high-throughput analysis of many samples
- Good linearity and sensitivity promote accurate quantification of varying concentrations of compounds in fermentation broth samples (Table 2)
- Small amount of a minimally prepared sample is needed to achieve accurate quantification
- Non-contact sampling method ensures low carryover between samples (Figure 2).
Methods
Sample preparation: Standards and fermentation broth samples of all compounds were diluted with methanol:water (1:1). After the fermentation broth supernatant was diluted 10,000 times, 50 µL was added to a 384-well sample plate.
Acoustic ejection method: The sonic excitation ejection method requires a suitable carrier solvent to bring the sample droplets into the OPI and then into the ion source. In this experiment, methanol containing 0.05% formic acid was selected as the carrier solvent, with an optimal flow rate of 360 µL/min and ejection volume of 25 nL.
Mass spectrometry: The Echo MS system was coupled with the SCIEX Triple Quad 6500+ system. SCIEX OS software was used to control the system. MRM transition information is outlined in Table 1. Transitions were split into 3 acquisition methods, 1 method in positive ion mode and 2 in negative ion mode.
Table 1. Compound information. MRM conditions for each of the analytes in the assay, split across 3 acquisition methods.
Table 2. Assay quality. Concentration of the standard curve, linearity, reproducibility and spike recovery for all compounds are shown.
Linearity and recovery
On the Echo MS system, the 10 compounds were analyzed by MRM acquisition, using 3 MS methods. Concentration curves were generated across 12 different concentrations for each standard. Summary data are presented in Table 2. Figure 2 shows an example of the raw data for 1,5-diaminopentane, which includes the spectrum of 6 consecutive ejections of each standard curve point, blank solvent and matrix sample. Reproducibility was assessed by measuring 6 ejections at each concentration (Figure 2). All 10 compounds had good reproducibility within the range of the standard curve. Even at the lower limit of quantification (LLOQ), the reproducibility for 6 consecutive ejections was less than 12%. The linearity of the 10 calibration curves was greater than 0.99 across the 3.5 orders of concentration evaluated. Examples are shown in Figure 3.
To assess carryover, 6 consecutive ejections were analyzed at a high concentration of 500 µM, followed by 6 blank ejections. Figure 2 shows that there is no signal detected in the blank ejections that follow the 500 µM sample, indicating that there is minimal carryover between ejections.
A spike recovery experiment was performed using a sample matrix spiked with each standard at a 5 µM concentration. The spike recovery rate of all compounds was greater than 80%, demonstrating minimal matrix suppression effects on the system. Table 2 provides linear range, repeatability and recovery information for all compounds tested.
Figure 2. Reproducibility of ejections in matrix. To demonstrate reproducibility, 6 repeat ejections of 1,5-diaminopentane were performed across the range of concentrations of the neat standard curve. Blank samples and the fermentation broth samples are shown.
Figure 3. Linearity and dynamic range. Four compounds were selected to demonstrate linear dynamic range (LDR) of the assay. Good linearity was observed across the 3.5 orders of LDR interrogated. |
Conclusions
The Echo MS system provides a rapid and high-throughput quantification method for 10 target compounds in fermentation broth. Each compound demonstrates excellent linearity and the recovery rate of spike experiments is greater than 80% without any observable carryover. These results show that the Echo MS system can produce high-quality results for the accurate quantification of amino acids, organic acids and pentamethylenediamine in the fermentation broth matrix, even with a simple sample preparation approach.
This method is accurate in quantification, high in throughput and simple in sample pre-processing. The entire experimental data set required only 1 hour of acquisition time to complete using the 3 methods on a 384-well plate of samples, and therefore is significantly faster than using a comparable rapid, 5-minute LC-MS method which would require more than 32 hours to complete this work. The Echo MS system can be used for rapid screening of strains in the early stage of microbial fermentation engineering and real-time monitoring and optimization of fermentation processes, thereby improving production efficiency.
References
- Rapid quantitative analysis of fermentation broth samples to assess efficiency of engineered yeast strain turnover. SCIEX technical note, RUO-MKT-02-11695-A.
- Rapid MS/MS analysis with Acoustic Ejection Mass Spectrometry (AEMS) - Using the SCIEX Echo® MS system to break bottlenecks in quantitative mass spectrometry throughput. SCIEX technical note, RUO-MKT-02-11385-A.
 Click to enlarge
Click to enlarge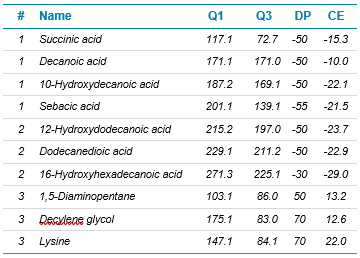 Click to enlarge
Click to enlarge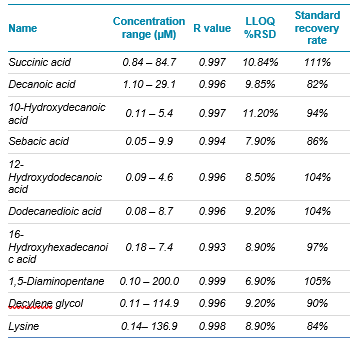 Click to enlarge
Click to enlarge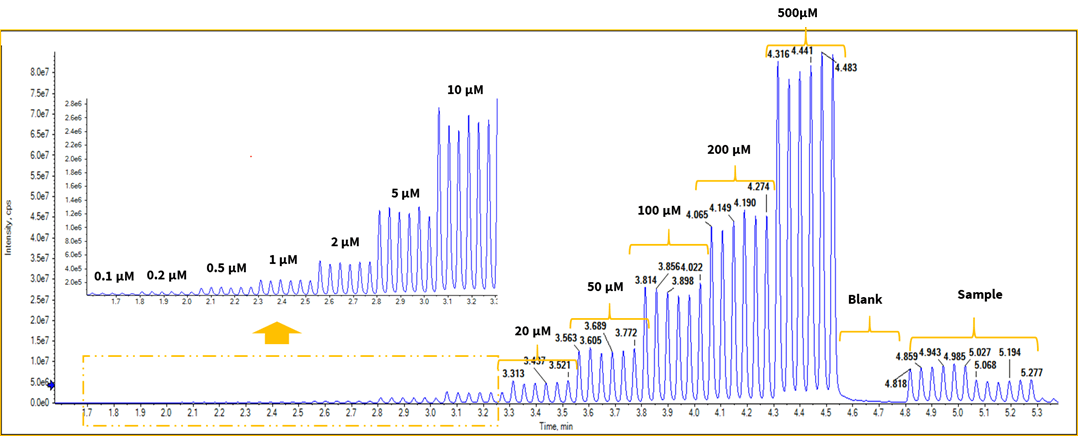 Click to enlarge
Click to enlarge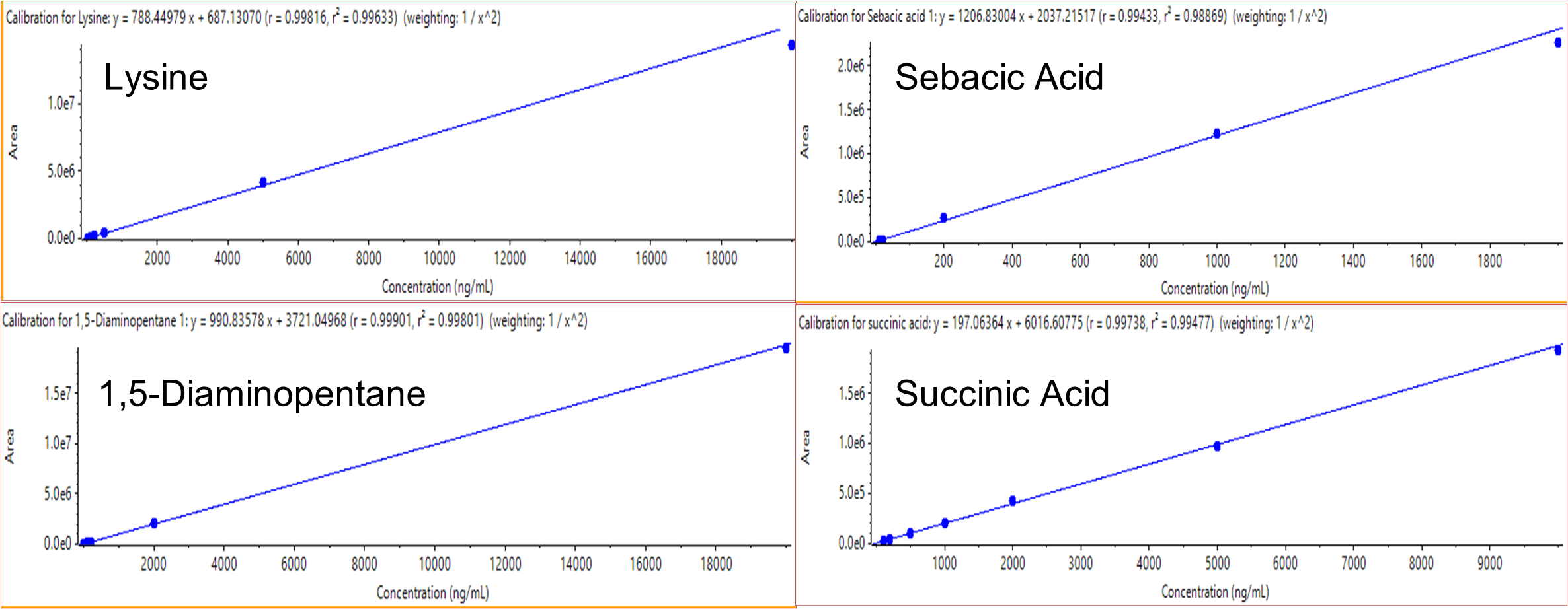 Click to enlarge
Click to enlarge