Methods
Sample preparation: NIST SRM 1950 plasma was extracted using 8 volumes of methanol then spiked with internal standard QReSS kit (Cambridge Isotopes). After centrifugation to precipitate the proteins, the supernatant was directly analyzed. More details on the sample preparation can be found in the SCIEX How method.4
Chromatography: Samples were analyzed using the ExionLC system with a Kinetex F5 column (2.1 x 150 mm, 2.6 µm, Phenomenex). A simple linear gradient from 0 to 95% B was used with standard reversed phase mobile phases (A = 0.1% formic acid in water and B = 0.1% formic acid in acetonitrile) with a flow rate of 200 µL/min. A 1 µL injection volume was used and the column temperature was maintained at 30 ºC throughout the analysis. Download the full protocol for more details.4
Mass spectrometry: The sample extract was analyzed on both the QTRAP 6500+ system and the SCIEX 7500 system. Data were collected using methods built with the Scheduled MRM algorithm as well as unscheduled methods. Finally, this high throughput method is used to analyze 336 serum metabolites (a total of 784 MRM transitions) in a 20 min run period. More details on the MS settings and the MRM transition list can be downloaded from the SCIEX How method.4
Data processing: All data was analyzed using the Analytics module in SCIEX OS software 2.0.
Chromatography
Chromatography of metabolites can be difficult due to the broad range of properties across the compounds of interest. Balancing the retention of polar metabolites as well as separation across the gradient of many other classes of metabolites is key to the development of a generic global profiling method. A reverse-phase method with a Kinetex F5 column (Phenomenex) was used to retain both nonpolar and polar analytes in a single separation for this broad targeted metabolite assay. Figure 3 highlights the separation achieved for the metabolites detected in positive ion mode and negative ion mode, color coded by metabolite class. The retention time reproducibility was highly consistent across the 10 replicates, with an RSD of 0.38% in plasma matrix.
It should be noted that the method described here is intended for use as a starter method to cover a broad range of metabolites, metabolite classes and associated pathways. Optimization of the chromatography, compounds monitored, and the specific MRM transitions and parameters could quickly be performed depending on the specific matrix, pathways, and analytes of interest.
Importance of time scheduling MRMs
Injections were performed without time scheduling in positive (blue) and negative (green) ion polarities to detect metabolites and determine retention times. Replicate injections using Scheduled MRM™ Algorithm were then performed in positive and negative ion mode to further refine retention times. The increased dwell times afforded by the use of the Scheduled MRM Algorithm provide improved peak area reproducibility across 10 replicates, as seen in Figure 4.
After removal of a small number of transitions that were poorly detected in this particular plasma sample, 264 MRM transitions were monitored in positive mode for the final dataset. A large improvement in peak area reproducibility was observed with time scheduled (blue solid) and without time scheduled (blue dotted). In the unscheduled method, a dwell time of 5 msec was set, giving a cycle time of 1.8 sec. After time-scheduling, the average dwell time was 64 msec and the target cycle time was set at 1 sec.
A more dramatic effect was observed in negative mode where a much larger number of MRM transitions (470 final MRM transitions) was monitored (Figure 4, green lines). Again, a large increase in dwell time is obtained upon using the Scheduled MRM Algorithm for scheduling such a large numbers of MRM transitions, hence the increase in data quality and reproducibility. In this case, with many more analytes, a dwell time of 5 msec was used, giving a cycle time of 3.3 sec for the unscheduled method (dotted green lines). After time-scheduling, the average dwell time was 20 msec and the target cycle time was set at 1 sec (solid green lines).
Fast polarity switching
The next step was to combine both the positive mode and negative mode analytes together into a single, final method. This was possible due to the fast polarity switching available on the SCIEX 7500 System. A total of 734 MRM transitions were combined into the final single, time-scheduled MRM assay with polarity switching, to get wide metabolite coverage in a single assay. Ten replicate injections were performed and the peak area reproducibility was compared to the previous single polarity methods (Figure 5, red line). High data quality was maintained when running the complete assay. There is no decay in the cumulative reproducibility curve from what was seen in negative mode only (470 MRMs) when compared to the full polarity switching method (734 MRMs).
In this final dataset, ~70% of analytes were reproducibly measured across 10 replicates with %CV < 10%, and ~85% were measured (or in other words, about 600 MRM transitions) with %CV < 20%. The final average dwell time was 14 msec and the target cycle time was set at 1 sec.
Dynamic range and coverage
The ability to precisely quantify both the high abundant metabolites as well as the low abundant analytes in a large multiplex panel is essential for researchers. This assay covers a wide range of metabolites in plasma spread across a broad range of abundance (Figure 6, top). On both the QTRAP 6500+ System and the SCIEX 7500 System, the instrument dynamic range is very high, up to 6 orders of linear dynamic range, enabling this large assay to be performed in a single run using simple sample preparation. The reproducibility observed for the higher abundant analytes was below 5% CV in the final assay. As expected, reproducibility decreases slightly as the abundance decreases, but still remains good down to the very low abundant, difficult to detect metabolites (Figure 6, bottom).
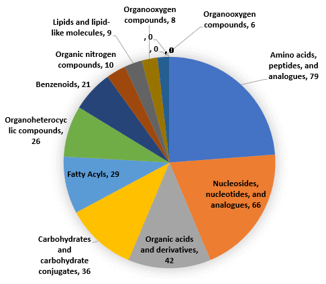
Finally, the method encompasses compounds from 69 pathways including the TCA cycle, glycolysis, purine pyrimidine metabolism and amino acid metabolism (Figure 7).
Conclusions
The large scale polarity switching method using Scheduled MRM Algorithm has been developed to quantify ~336 serum metabolites in plasma and can be used for large scale targeted metabolomics studies.
- The sMRM Pro Builder Tool was developed to streamline optimization of the sMRM assay and provide insights on MRM assay data quality
- Time scheduling of MRMs provided significant improvements over unscheduled methods for this large of a number of MRM transitions, with 95% of positive mode data and 80% of negative mode data having peak area %CV ≤15%
- Using polarity switching to include both positive and negative ion mode metabolites in a single sMRM method also provided high quality data, with no loss in peak area reproducibility compared to individual single polarity sMRM method
- Improved sensitivity of the SCIEX 7500 system showed up to a 3-5 fold increase in signal to noise ratio over the QTRAP 6500+ system, which provides detection and quantification of ~30% more metabolites in plasma.3

 Click to enlarge
Click to enlarge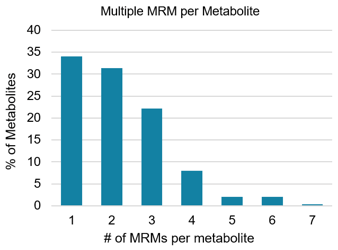 Click to enlarge
Click to enlarge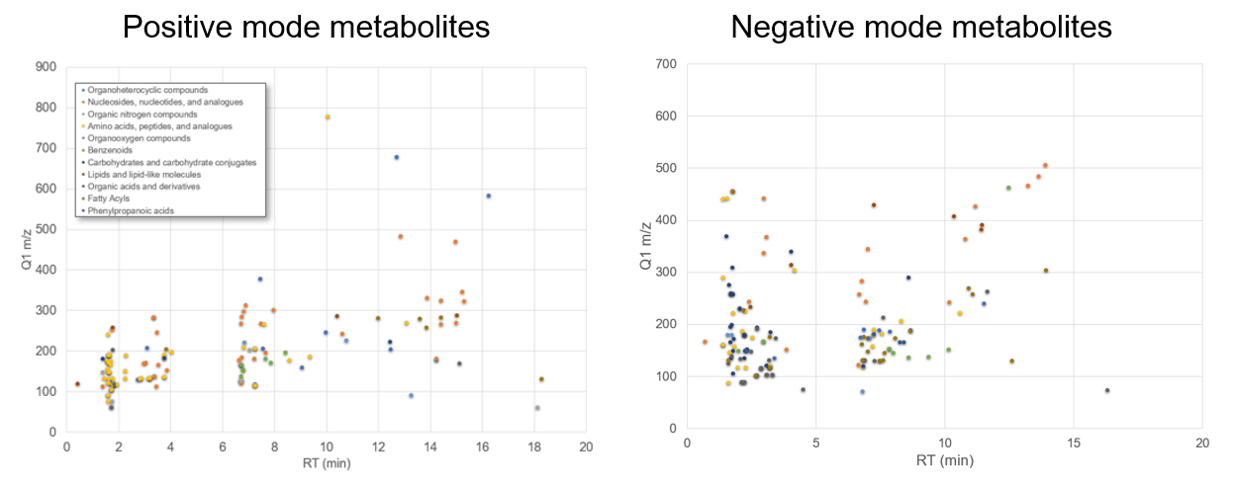 Click to enlarge
Click to enlarge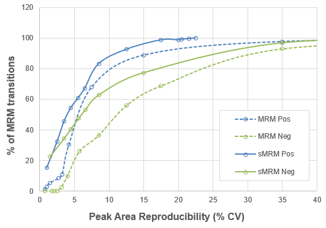 Click to enlarge
Click to enlarge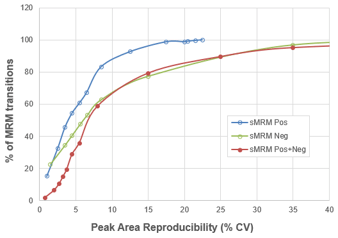 Click to enlarge
Click to enlarge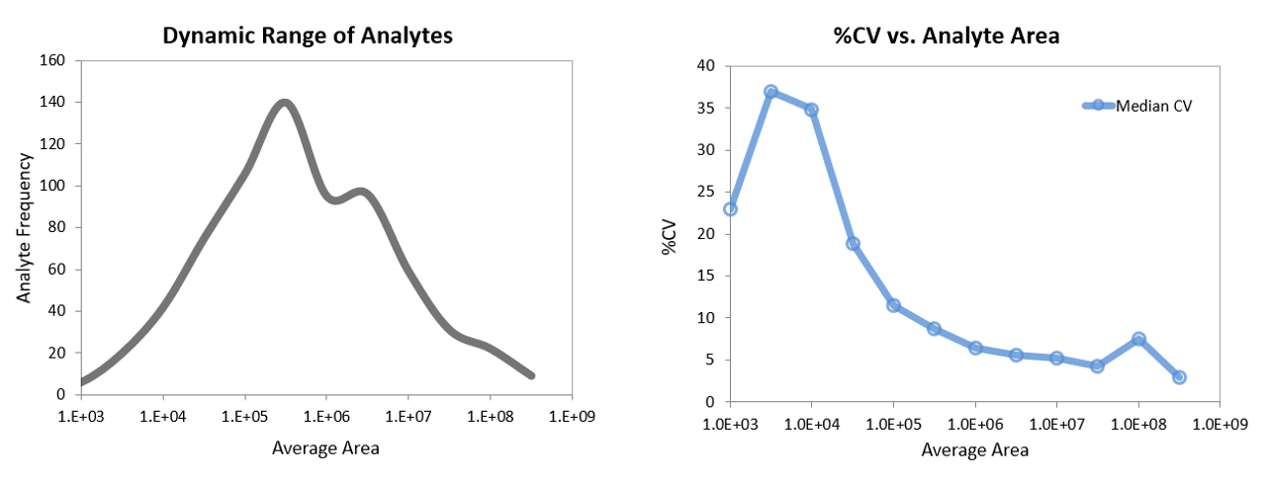 Click to enlarge
Click to enlarge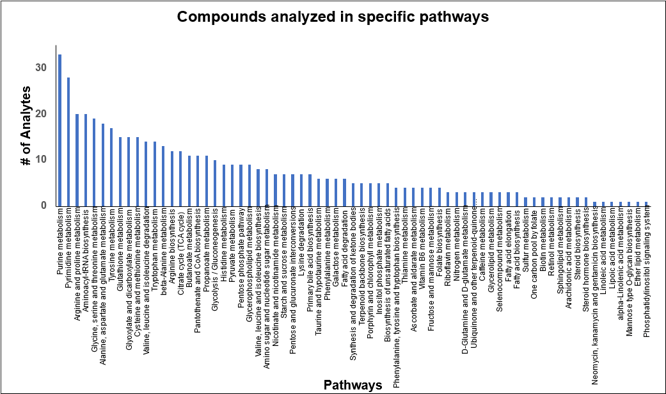 Click to enlarge
Click to enlarge