Abstract
Fluticasone propionate, a synthetic glucocorticoid with potent anti-inflammatory activity, requires very sensitive assays for monitoring pharmacokinetic parameters due to the very low therapeutic inhaled dose ranges. This has required use of large sample volumes. Here, a selective, sensitive and reproducible bioanalytical method was developed for quantitation of fluticasone propionate (LLOQ of 200 fg/mL) in human plasma with the QTRAP 6500 System. Reduced sample volume (500 µL plasma) and a final reconstitution volume of 200 µL for reinjection of samples or repeat analysis chromatography if required in a GLP laboratory.
Introduction
Fluticasone propionate is a synthetic glucocorticoid with potent anti-inflammatory activity effectively used in the treatment of chronic asthma and obstructive pulmonary diseases. It is mainly administered via inhalation because of its negligible (<1%) oral systemic bioavailability. Plasma concentrations of fluticasone propionate at therapeutic inhaled dose ranges are extremely low and require sensitive assays to determine the pharmacokinetic parameters. Systemic exposure data of fluticasone propionate, 88 mcg twice daily dose after inhalation in adults and adolescents (>12 years) shows an average Cmax of 20 pg/mL which necessitates the use of a sensitive analytical method that can quantify at sub-pico gram per mL levels.
In recent years, many analytical methods have been developed for pharmacokinetic studies or clinical trials of fluticasone propionate, however, to achieve the necessary sensitivity, most of the methods use a large plasma sample aliquot and a low reconstitution volume which limits the feasibility of performing reinjection reproducibility or repeat analysis in a GLP regulated bioanalytical laboratory. This is also a concern for regulatory compliance of clinical studies.
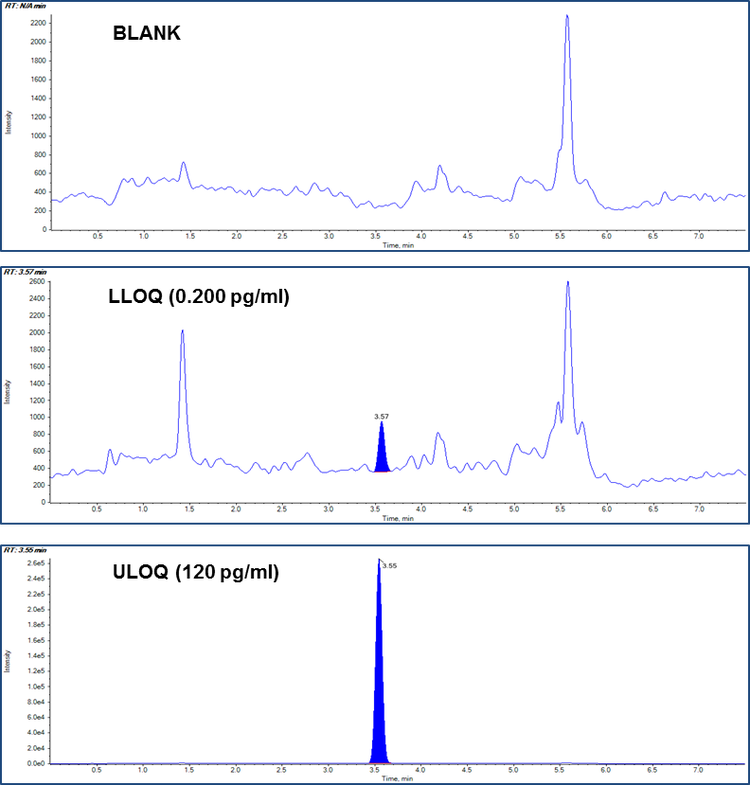
The main objective of this work is to develop a sub-pico gram level LOQ (200 fg/mL) LC-MS/MS method, feasible for a regulated bioanalytical laboratory, for the quantification of fluticasone propionate in human plasma using fluticasone propionate –D3 as internal standard (Figure 1).
Key features of this targeted method
- A highly sensitive and reproducible method for quantifying fluticasone propionate was developed for use in regulated bioanalytical labs using a simple solid phase extraction technique.
- The LLOQ for fluticasone propionate in plasma was 0.200 pg/mL with an aliquot volume of 500 µL of plasma.
- Patented IonDrive™ Technology provides improved ion production, transmission and detection for ultimate sensitivity and reproducibility.
- Next-generation eQ™ Electronics on the QTRAP 6500 System provide improved performance at ultra-low MRM dwell times for improved support of fast LC and narrow peak widths.
Methods
Sample Preparation: Fluticasone propionate standards were prepared in Human K2EDTA plasma from 0.200 to 120 pg/mL and QC samples were prepared at 0.200, 0.600, 60.0 and 100 pg/mL. Samples (500 µL) were spiked with 50 µL of fluticasone propionate-D3 (25 pg/mL) as internal standard solution and were subjected to protein precipitation followed by reverse phase SPE purification using Cleanert S C18-SPE cartridge. After loading, the samples were washed with water followed by 25% methanol twice and then eluted using dichloromethane. The eluent was dried under a stream of nitrogen at 40ºC. Dried samples were then reconstituted with 200 µL of mobile phase for LC-MS analysis.
Mass Spectrometric Conditions: The SCIEX QTRAP 6500 System with the IonDrive Turbo V source was operated in positive electrospray ionization mode. The MS conditions were as follows: scan type positive MRM, Q1 resolution at unit and Q3 at unit; curtain gas set at 25; ion source temperature 400°C, ion source gas (GS1) at 75 and drying gas (GS2) at 70; ion spray voltage at 3000 V; and dwell time 200 msec for all transitions. The compound dependent parameters for analyte and internal standard used are outlined in Table 1.
Data Processing: Analyst® Software 1.6 was used for mass spectrometer data acquisition and MultiQuant™ Software 3.0.2 was used for processing. A 1/x2 weighted linear regression was used to calculate the concentrations.
Results
Fluticasone propionate produced two intense product ions at m/z 293.2 and 313.2 and both were selected for quantitation using summation of peak area response. The fluticasone propionate-D3 (IS) product ion at 313.2 was selected for the internal standard (Table 2).
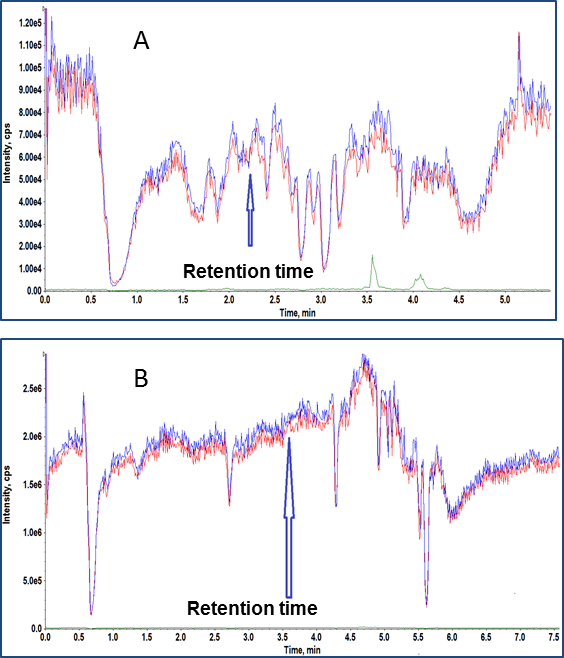
Various sample extraction techniques like liquid-liquid extraction, protein precipitation and SPE were tested to improve the separation of the analyte of interest from the matrix components. The SPE technique outlined above produced promising results over the published sample prep methods because it removed a significant number of matrix components as evidenced by the zone free of matrix suppression (Figure 2A & 2B) at the analyte and IS retention time.
In the present method, linearity was established in the range of 0.200 to 120 pg/mL in human plasma. The calibration curve is shown in Figure 3 with correlation coefficient r = 0.99. Table 3 shows the accuracy and precision data at different QC levels of fluticasone propionate. All are within the acceptance criteria of %CV ±20% at LLOQ level and ±15% at other levels. Example chromatograms of the blank, LLOQ and ULOQ calibration standards are shown in Figure 1.
Conclusions
A highly selective, sensitive and reproducible bioanalytical method was developed for the detection of fluticasone propionate with an LLOQ of 200 fg/mL in human plasma with the QTRAP 6500 System. A key property of this method is the required sample volume (500 µL plasma), which is lower than most other methods plus the final reconstitution volume of 200 µL makes this method amenable for reinjection of samples or repeat analysis chromatography if required in a GLP laboratory.
References
- Allena J, et al. (2013) Ultrasensitive and automated 1 pg/ml fluticasone propionate assay in human plasma using LC-MS/MS. Bioanalysis 5(4): 423-35.
- Krishnaswami S, et. al. (2000) A sensitive LC–MS/MS method for the quantification of fluticasone propionate in human plasma . J. Pharm. Biomed. Anal. 22, 123–129.
- Byrro RM et al. (2012) A rapid and sensitive HPLC-APCI-MS/MS method determination of fluticasone in human plasma: application for a bioequivalency study in nasal spray formulations. J Pharm Biomed Anal. 5;61: 38-43.




