Analysis of nitrosamine compounds in multiple sartan APIs: a review and optimization of the Ph. Eur. monograph
Using the SCIEX QTRAP® 4500 LC-MS/MS System with the ExionLC™ AD System
Jack Steed1, Sandeep Choudhary2, Pankaj Partani2
1SCIEX, UK; 2SCIEX, India
Introduction
Nitrosamine analysis has recently become one of the largest areas of interest in the pharmaceutical industry after their production in numerous active pharmaceutical ingredients (APIs) and drug products was found to be possible. Because nitrosamines are believed to be genotoxic, their strict regulation is necessary to ensure human health is not adversely affected.
The interest in nitrosamines began in 2018, when it was realized that nitrosamine formation might be occurring in various sartan APIs. The concern subsequently moved onto ranitidine and metformin, with several other pharmaceuticals also being called into question. This then provides an analytical challenge because of the speed at which the issue has grown since the first introduction in 2018, and the various products that have been implicated. Therefore, robust and sensitive analytical methods are necessary due to the relatively low limits that have now been provided as guidance by both the FDA and EMA1-2. These limits currently stand at either 26.5 ng/day or 96 ng/day depending on the nitrosamine compound of interest. This equates to a general limit of 30 ng/g for drug products which have a maximum daily dose (MDD) below 880 mg/day, with adjustment necessary if this MDD is exceeded.1
Here, three different methods have been provided that aim to improve upon the recently released European pharmacopeia chapter 2.5.42, N-nitrosamines in active substances, by optimizing run time, expanding the number of nitrosamine compounds analyzed and increasing sensitivity.2 The reasons for three different methods are the complexity of analyzing various APIs by one method and the need to alter the gradient to either include additional nitrosamine compounds or to cover the entire range of APIs tested.
All of the analysis described here has been performed on the SCIEX QTRAP 4500 LC-MS/MS System, coupled to an ExionLC AD System. This system was used because it is able to achieve the current limits within sartan APIs and can be utilized for routine analysis even at challenging limits of detection and quantification.
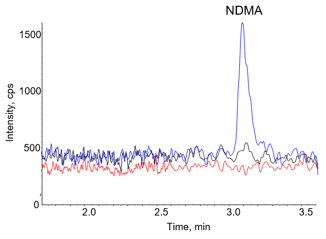
Figure 1. Detection of NDMA in Candesartan API. Overlaid XICs highlighting the NDMA quantifier ion of 75 > 58 M/Z for a spiked (0.45 ng/mL) 30 mg/mL Candesartan API preparation (blue), an unspiked 30 mg/mL Candesartan API preparation (black) and a water blank (red). |
Key features of the SCIEX QTRAP 4500 LC-MS/MS System
- Sensitivity to meet the current established limits for nitrosamines in various sartan APIs
- The robustness necessary to analyze large numbers of samples
- High levels of specificity by utilizing both quantifier and qualifier transitions
Table 1. A list of sartan APIs analyzed.
Methods
Standard preparation: Each nitrosamine impurity was weighed or pipetted before being diluted to achieve a mixed standard solution with a concentration of 9 ng/mL.
Sample preparation: 150 mg of API was weighed into a suitable vessel before 1 mL of methanol was added. The solution was mixed thoroughly for ~ 5 minutes before being sonicated for 15 minutes. 4 mL of water was added before being mixed thoroughly for ~ 5 minutes and then sonicated for 15 minutes. The supernatant was removed and filtered through a 0.20 µm PTFE filter prior to analysis.
Spiked sample preparation (0.90 ng/mL spike): 150 mg of API was weighed into a suitable vessel before 0.5 mL of nitrosamine standard solution (9 ng/mL) was added along with 0.5 mL of methanol. The solution was mixed thoroughly for ~ 5 minutes before being sonicated for 15 minutes. 4 mL of water was added before being mixed thoroughly for ~ 5 minutes and then sonicated for 15 minutes. The supernatant was removed and filtered through a 0.20 µm PTFE filter prior to analysis.
Spiked sample preparation (0.45 ng/mL spike): 150 mg of API was weighed into a suitable vessel before 0.25 mL of nitrosamine standard solution (9 ng/mL) was added along with 0.75 mL of methanol. The solution was mixed thoroughly for ~ 5 minutes before being sonicated for 15 minutes. 4 mL of water was added before being mixed thoroughly for ~ 5 minutes and then sonicated for 15 minutes. The supernatant was removed and filtered through a 0.20 µm PTFE filter prior to analysis.
Chromatography: Separation was performed using the ExionLC AD System. See supplementary information for more detail of the methods.3
Mass spectrometry: The QTRAP 4500 System was operated in positive polarity using atmospheric pressure chemical ionization (APCI). See supplementary information for instrument settings, MRM transitions and source conditions for both methods.3
Initial method results
Initially, five nitrosamine compounds were analyzed, in line with the European pharmacopeia (chapter 2.5.42) (see Table 2 below for a list of compounds analyzed), at a concentration of 0.45 ng/mL. This provided a limit of 0.015 µg/g after taking sample preparation into account. Each API was individually spiked at this level and analyzed across 6 replicates (Figure 2). The observed reproducibility, the %CV values, are provided for each compound in Table 2. All nitrosamines analyzed on this method provided acceptable reproducibility within the 5 APIs, measured with %CV values <15% and S/N values >10x. The limit of 0.015 µg/g was initially chosen to highlight that the SCIEX QTRAP 4500 LC-MS/MS System is capable of easily achieving the limit of 0.03 µg/g in all APIs analyzed. All subsequent analysis was at the specification limit of 0.03 µg/g (0.9 ng/mL) to more closely resemble the levels which would typically be expected when following the European pharmacopeia method.
Table 2. Representative data of a 30 mg/mL Candesartan API, spiked at 0.45 ng/mL with each nitrosamine compound listed.
Figure 2. Separation achieved for nitrosamines in Candesartan Cilexetil. Chromatography of a 30 mg/mL Candesartan Cilexetil API spiked at 0.45 ng/mL with the five nitrosamine compounds outlined in Table 2.
Updated method for additional nitrosamines
After the testing performed on the initial method, an attempt was made to add additional nitrosamine compounds (see Table 3 for full list of compounds analyzed). In doing this, however, it was realized that the initial gradient profile did not provide adequate separation of N-nitrosodibutylamine (NDBA) with some of the APIs tested. This meant that an elongation of the method was necessary to provide this separation. See Figure 3 for representative chromatography of a 0.9 ng/mL spiked Candesartan Cilexetil API, when using this gradient.
Table 3. Nitrosamine compounds analyzed on the updated and losartan specific methods.
Figure 3. Separation with higher spike level. Chromatography of a 30 mg/mL Candesartan Cilexetil API spiked at 0.9 ng/mL with the nitrosamine compounds outlined in Table 3. It is noted that due to NDIPA and NDPA being positional isomers, the separation is based purely on chromatography alone.
Losartan specific method
The final method used was due specifically to losartan’s co-elution with NDBA which proved to be the most challenging API to analyze because of this close elution on the analytical column used (C18 stationary phase). Therefore, the run time of the method needed to be slightly extended again from 35 minutes in the updated method to 40 minutes here. This was specifically for losartan (Figure 4), with the other APIs being analyzed solely on either the initial or updated gradient, depending on the nitrosamine compounds that needed to be analyzed.
Figure 4. Separation achieved with longer gradient. Chromatography of a 30 mg/mL losartan API spiked at 0.9 ng/mL with the nitrosamine compounds outlined in Table 3 using the losartan only gradient.
Conclusions
The methods outlined here meet and improve on the specifications outlined in the recently released European pharmacopeia chapter (increased sensitivity and additional compounds of interest) on nitrosamines and highlight the sensitivity, selectivity and robustness of the SCIEX QTRAP 4500 LC-MS/MS System. The methods display the flexibility of the system for analyzing various nitrosamine compounds in multiple APIs. The specification limit of 0.03 µg/g can be readily met while also achieving acceptable precision and signal to noise values.
References
- Control of nitrosamine impurities in human drugs – guidance for industry. FDA, September 2020.
- N-nitrosamines in active substances. European pharmacopeia chapter 2.5.42, December 2020.
- Download Supplementary information.

 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge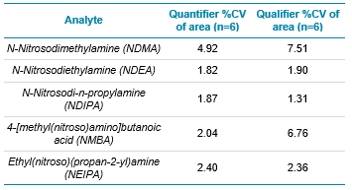 Click to enlarge
Click to enlarge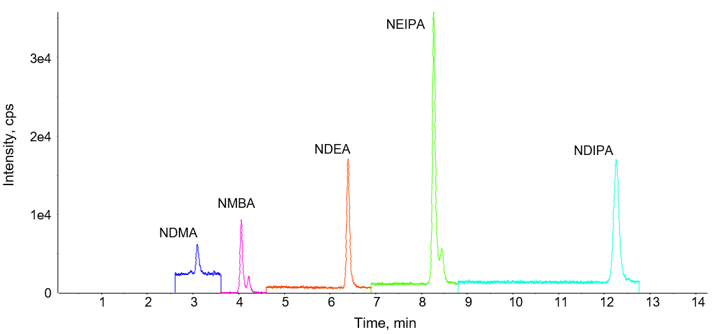 Click to enlarge
Click to enlarge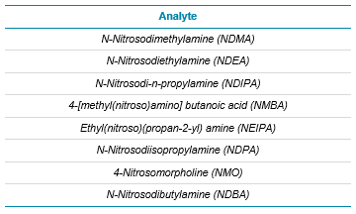 Click to enlarge
Click to enlarge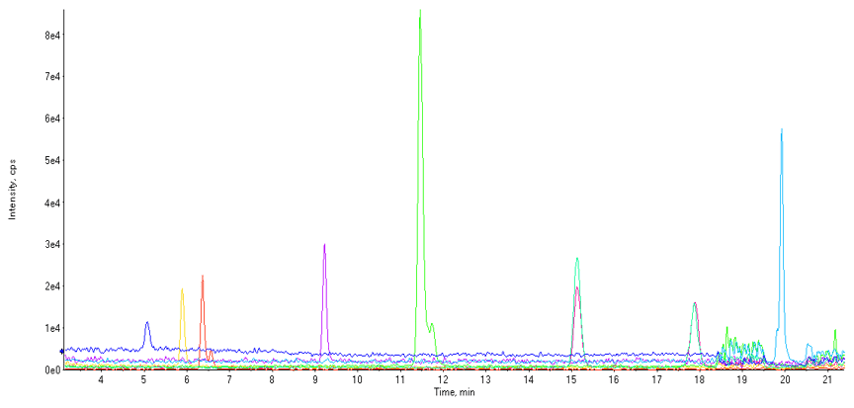 Click to enlarge
Click to enlarge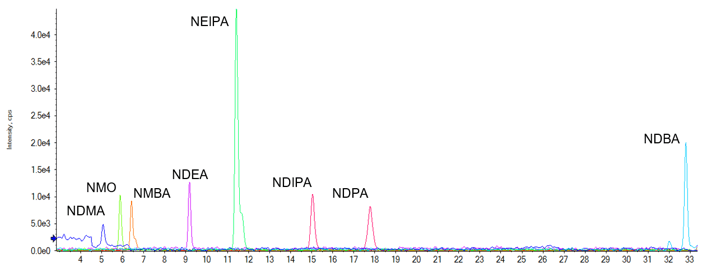 Click to enlarge
Click to enlarge