MRM3 quantitation for highest selectivity in complex matrices
Innovations on the QTRAP systems
Christie Hunter
SCIEX, USA
Abstract
Multiple Reaction Monitoring (MRM) experiments are the most common LC-MS/MS method used for high sensitivity robust quantitation in complex matrices. While much of the time, the MRM approach provides the required sensitivity, sometimes detection can be impacted by high background or interferences from the matrix. The unique hybrid triple quadrupole linear ion trap configuration of the QTRAP system enables a novel workflow termed MRM3 which can provide an added level of specificity to a quantitative assay when required. The fundamentals of the workflow are described here.

Introduction
Mass spectrometry has transformed quantitative analysis to become the method of choice for many assays. More recently, LC-MS/MS has revolutionized quantitative bioanalysis. While single MS filtering offers advantages over non-mass selective techniques, the use of tandem mass spectrometry (MS/MS, or MS2) eliminates interferences and results in a dramatic increase in selectivity which yields a very low baseline, excellent limits of quantification, and very good linearity. As a result, the Multiple Reaction Monitoring (MRM) experiment performed on triple quadrupole mass spectrometers has become the technique of choice for highly sensitive and selective quantification in biological matrices.
Mass spectrometry has transformed quantitative analysis to become the method of choice for many assays. More recently, LC-MS/MS has revolutionized quantitative bioanalysis. While single MS filtering offers advantages over non-mass selective techniques, the use of tandem mass spectrometry (MS/MS) eliminates interferences and results in a dramatic increase in selectivity which yields a very low baseline, excellent limits of quantification, and very good linearity. As a result, the Multiple Reaction Monitoring (MRM) experiment performed on triple quadrupole mass spectrometers has become the technique of choice for highly sensitive and selective quantification in biological matrices.
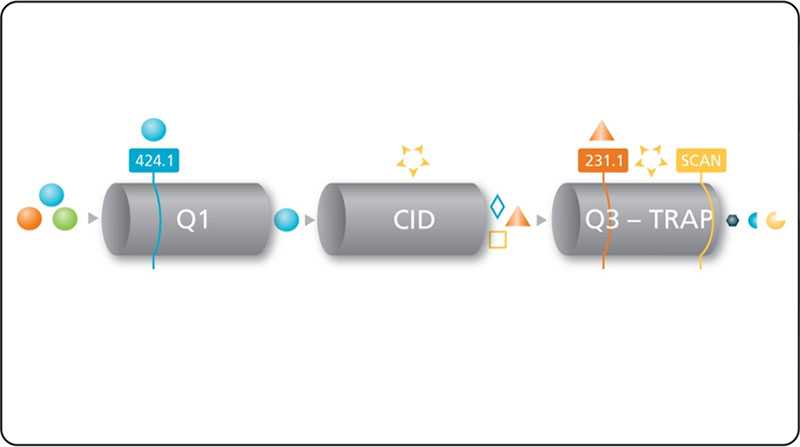
Figure 1. MRM3 for quantitative analysis by LC-MS. Analyte ion is first selected in the Q1 quadrupole, then fragmented in Q2 collision cell. Fragment ions are trapped then isolated in the linear ion trap, followed by excitation to perform the second fragmentation step. Second generation product ions are scanned out to the detector.
Key features of the QTRAP systems for MRM3 quantification
- MRM3 quantification - An MS3 scan is performed with a fast cycle time and using a narrow scan range centered at the second generation product ion m/z which is being used for quantification. This type of scan is referred to as MRM3 (Figure 1).
- Faster linear ion trap scan speeds – Scan speeds up to 20,000 Da/sec enable MS3 scans with an HPLC compatible cycle time such that extracted ion chromatograms (XICs) of second generation product ions can be extracted and integrated with a sufficient number of data points across the chromatographic peak.
- Better in-trap fragmentation – the Linear Accelerator trap with pulsed gas valve implemented in the QTRAP systems provides faster, more efficient in-trap fragmentation (Figure 2)
- High ion trap sensitivity – The QTRAP systems feature high sensitivity when operating in linear ion trap mode
- High selectivity – Unit isolation of precursor ion in Q1 followed by excitation and fragmentation at unit resolution in the ion trap provides the very high selectivity in MRM3 analysis (Figure 3).
Speed
The speed and efficiency of ion-trap fragmentation has also been greatly enhanced on the QTRAP systems. Collision gas is now introduced through a high speed pulsed gas valve that enables a rapid increase in pressure in the LIT (Figure 2). Together with an increase in the RF drive frequency, this pulsed gas results in increased fragmentation efficiency and reduced excitation time of 25 ms or less.
In addition, the scan speed of the linear ion trap has been increased to a maximum of 20,000 through the use of the faster eQ electronics. This enabling fast MRM3 scan cycles at very high sensitivity.
Selectivity
Because of the configuration of the QTRAP systems, the MRM3 quantification workflow is highly flexible and leverages the sensitivity and selectivity of the system. For the MRM3 workflow, the analyte ion of interest is first isolated in the Q1 quadrupole with a user–selected resolution, usually unit resolution (0.7 Th FWHH). It is then fragmented in the Q2 collision cell, providing a broad range of product ions to be selected in the ion trap.
The in-trap fragmentation is achieved through the application of a single wavelength / narrow band excitation. As shown in Figure 3, this allows very selective fragmentation. The C12 isotope of a product ion can be specifically excited and fragmented to completion with minimal fragmentation of the C13 isotope. This provides further selectivity advantages in the removal of interfering background.
Figure 2. Pulsed gas valve. Gas is introduced into the linear ion trap using a high speed pulsed gas valve which rapidly increases LIT pressure and reduces required excitation time for ion trap fragmentation.
Figure 3. Single frequency excitation for high selectivity. Narrow band excitation is used to specifically excite and fragment just the C12 isotope of the ion isolated in the LIT (left). This isotope can be fragmented to completion with no impact on the nearby C13 isotopes (right).
Sensitivity
Linear Accelerator trap technology has resulted in ground breaking improvement in the handling of ions inside the linear ion trap of the QTRAP systems, resulting in up to 100x more sensitivity. Trapped ions are manipulated within the linear ion trap through the use of auxiliary DC fields provided by the addition of small electrodes (Figure 4, top). Ions are gently moved toward the extraction region of the linear ion trap during the cooling period by a voltage applied to the trap collar. A potential barrier is created by increasing the potential on the auxiliary electrodes just before the mass scan to complete the ion concentration process. The application of this axial field has a significant effect on sensitivity (Figure 4, bottom left).
In addition, a radio frequency is applied to the exit lens of the Linear Accelerator Trap resulting in further sensitivity gains (Figure 4, bottom right). These two innovations enable better than unit resolution to be obtained in the trap scan modes at these very high scan speeds.
Figure 4. Linear Accelerator trap Innovations for sensitivity. Addition of electrodes in this new trap design (top) significantly improves the sensitivity of trap scanning by moving the trapped ions into the extraction region before axial ejection from the trap (bottom left). Further sensitivity gains are achieved by the addition of RF to the exit barrier of the trap (bottom right).
Removal of tough interferences
Innovations in scanning speed, selectivity and sensitivity on the QTRAP systems enable successful implementation of the MRM3 workflow for a wide range of analytes 3,4. Sometimes, background noise or interferences can limit the detection of an analyte. Shown in Figure 5 is an example of an interference that has the same MRM transition as Clenbuterol and elutes at the same retention time. Use of MRM3 can completely remove this interference and enable a much lower detection of this analyte.
Figure 5. Analysis of clenbuterol in urine. Analysis of Clenbuterol in urine by MRM is plagued by the presence of a large co-eluting interference. Left – MRM for Clenbuterol used to analyze the urine blank. Middle – MRM3 analysis of the urine blank shows the interference is completely gone. Right – MRM3 analysis of 0.5 ng/mL clenbuterol spiked into urine. 10x better LOQ obtained with MRM3 than MRM due to substantial reduction in interference (data not shown).
Conclusions
- MRM3 is an effective strategy for quantitation of analytes when high background or interferences make standard MRM quantitation difficult (Figure 5)
- MRM3 can be used to achieve similar LOQ‘s with less sample preparation and simplified or faster chromatography
- MRM3 has been successfully applied to the detection and quantitation of small molecules, peptides, and protein biomarkers
- MRM3 is significantly improved on the QTRAP systems, making them useful tools for quantitation in tough matrices
- The QTRAP systems are high performance triple quadrupole and linear ion trap systems providing users with many powerful quantitative and qualitative tools.
References
- Collings BA, (2007) Fragmentation of ions in a low pressure linear ion trap. J. Am. Soc. Mass Spectrom. 18, 1459-1466.
- Collings BA, and Romaschin AR, (2009) MS/MS of ions in a low pressure linear ion trap using a pulsed gas. J. Am. Soc. Mass Spectrom. 20, 1714-1717.
- Fortin T. et al, (2009) Multiple Reaction Monitoring Cubed for Protein Quantification at the Low Nanogram/Milliliter Level in Nondepleted Human Serum. Anal. Chem. 81(22), 9343.
- Niessen J. et al, (2009) Human platelets express OATP2B1, an uptake transporter for atorvastatin. Drug. Metab. Dispos. 37(5), 1129.
 Click to enlarge
Click to enlarge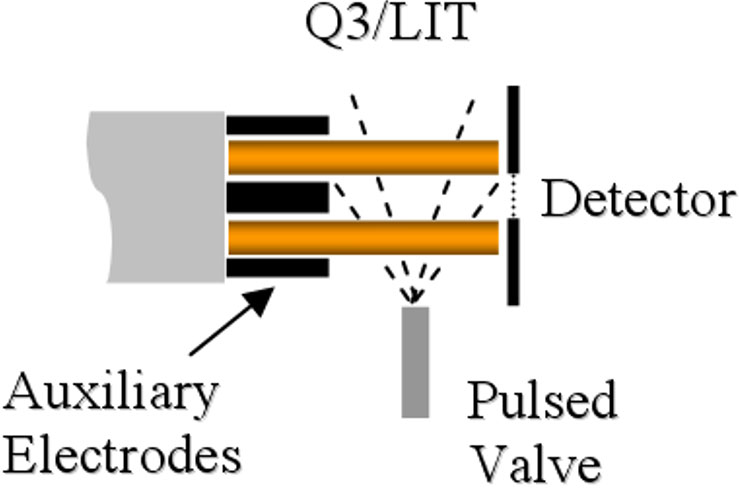 Click to enlarge
Click to enlarge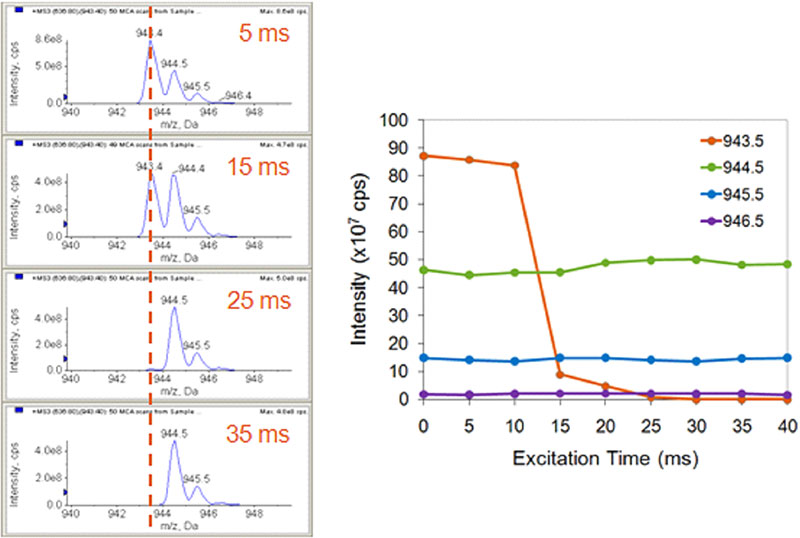 Click to enlarge
Click to enlarge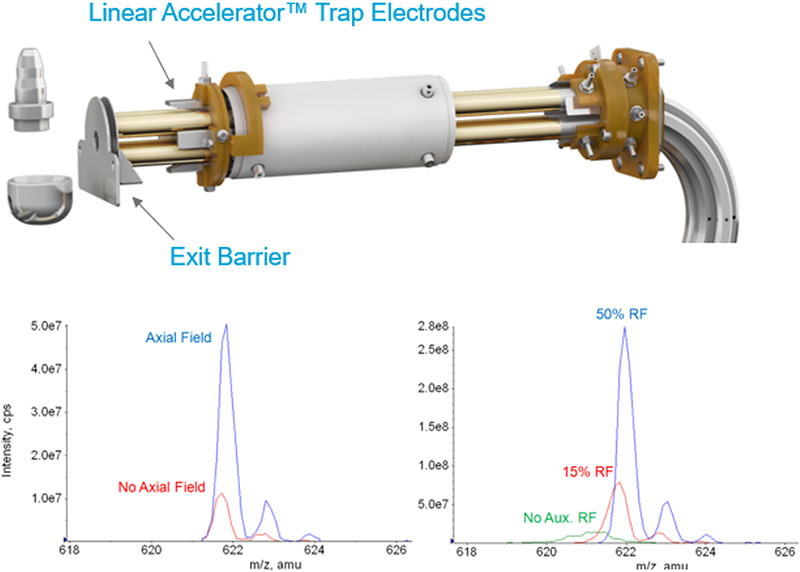 Click to enlarge
Click to enlarge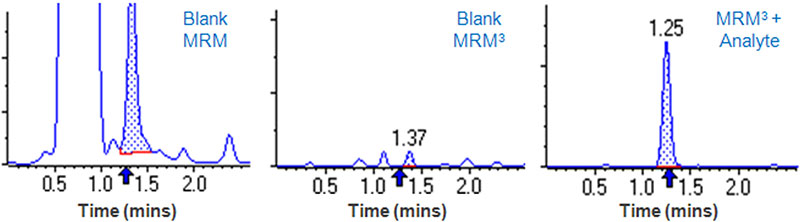 Click to enlarge
Click to enlarge