Differential Mobility Separation with LC-MS/MS for measurement of low-level testosterone in serum
Michael Jarvis1 , Dan Blake2
1SCIEX, Canada; 2SCIEX, UK
Abstract
Simple and rapid analysis of steroid hormones is often hindered by interferences and matrix effects, resulting in lengthy chromatography, extended extraction protocols and limited sensitivity. The use of Differential Mobility Separation (DMS) with the SCIEX SelexION Device can overcome these issues with enhanced analytical performance.

Introduction
It has been well documented that liquid chromatography-tandem mass spectrometry (LC-MS/MS) provides excellent accuracy, precision and sensitivity for measurements of steroids in biological matrices compared to traditional techniques such as immunoassays, which may suffer from cross-reactivity.
Nevertheless, there are numerous uncharacterized, endogenous components in biological fluids which have the potential to interfere with the measurement of low-level steroids such as testosterone. In this work, a novel method is presented employing differential mobility separation (DMS) in conjunction with LC-MS/MS analysis to eliminate potential interferences, thereby simplifying sample pre-treatment and enabling reduced LC run-times.
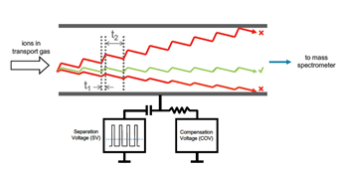
Liquid chromatography (LC), differential mobility separation (DMS), and tandem mass spectrometry (MS/MS) was used to enable the accurate quantification of low-level testosterone in human serum. The DMS cell filters out potential interferences prior to detection by MS/MS, ensuring that isobaric components do not obfuscate the analysis. The mechanism of DMS utilized in the SelexION®+ Differential Mobility Separation Technology is shown in Figure 1.
Figure 1. SelexION+ Differential Mobility Separation Technology. This device consists of two planar electrodes. Voltages are applied, which serve to filter out isobaric interferences prior to detection by tandem mass spectrometry.
Methods
Sample preparation: Testosterone and testosterone-d3 were obtained as 1mg/mL standards in methanol, from Cerilliant Corporation. Calibration curves were prepared by spiking known amounts of testosterone into steroid-free serum obtained from Golden West Biologicals.
Sample preparation consisted of a one-step liquid-liquid extraction. 200 µL of serum sample was combined with 50 µL of internal standard solution and 1000 µL of 90:10 hexane ethyl acetate in a micro-centrifuge tube. The sample was vortex mixed, centrifuged at 14,000 rpm for 15 minutes, and then 900 µL of the organic supernatant was removed and evaporated to dryness under a stream of nitrogen gas. The dried sample was reconstituted in 100 µL of methanol, and then further diluted with 100 µL of deionized water.
HPLC conditions: Chromatographic separation was accomplished using a SCIEX ExionLC™ HPLC System, with a Phenomenex Kinetex C18 column (100x2.1mm, 2.6µm), at a flow rate of 0.6 mL/min. Mobile phase A consisted of water with NH4F. Mobile phase B consisted of methanol. The run-time was 7 minutes.
MS/MS conditions: MS/MS detection was performed using the SCIEX Triple Quad™ 6500+ System equipped with IonDrive™ Turbo V Source and operated in electrospray ionization mode. Multiple Reaction Monitoring (MRM) mode was employed, with 2 MRM transitions monitored.
Optimization of SelexION+ Device parameters was performed using T-infusion of testosterone at mobile phase flow rate of 0.6 mL/min. At a fixed separation voltage (SV) of 3700 V, the compensation voltage (COV) was ramped across a broad voltage range using a step-size of 0.5V. The optimum COV value, producing a maximum in signal intensity, was observed at a value of 8.9 V as shown in Figure 2.
Figure 2. Optimization of COV parameters for testosterone.
Results
The limit of quantification (LOQ) was observed to be <1 pg/mL. Sensitivity and linearity are shown on a calibration curve in neat solvent in Figure 3.
To demonstrate the improved sensitivity when operating the ion mobility cell, a sensitivity comparison in neat solvent is presented in Figure 4. The total counts (cps) are lower when SelexION+ Technology is employed, however the S/N and LOQ are equivalent, or better, when the ion mobility device is used.
Figure 3. Linear calibration curve. The linear calibration curve was found to be linear from 0.1 – 100 pg/mL for testosterone in neat solvent, using SelexION+ Device.
Figure 4. Evaluation of detection limits. Comparison of LOQ and S/N, in neat solvent, with and without the SelexION+ Device.
The LC-DMS-MS/MS method was applied to the measurement of anonymized serum samples. As shown in Figure 5, the method displayed excellent sensitivity, and low chemical background due to the application of DMS.
Figure 5. Example data in matrix. Example data of testosterone analysis in serum is shown, showing zoom on y-axis, to visualize separation of interferences and low chemical background due to the use of the ion mobility filter.
Conclusions
The LC-DMS-MS/MS method presented here enabled the quantification of testosterone in human serum at <1pg/mL. No compromise in analytical sensitivity (LOQ) was observed when employing the ion mobility cell. This method provides the added advantage of improved specificity, and therefore the possibility of simplified sample preparation.
References
- SelexION® Technology: the solution to selectivity challenges in quantitative analysis. SCIEX technical note RUO-MKT-02-3251-C.
 Click to enlarge
Click to enlarge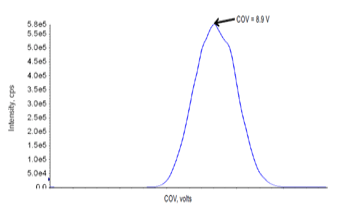 Click to enlarge
Click to enlarge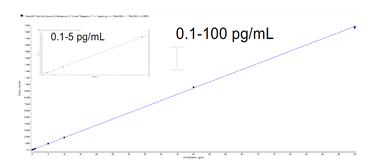 Click to enlarge
Click to enlarge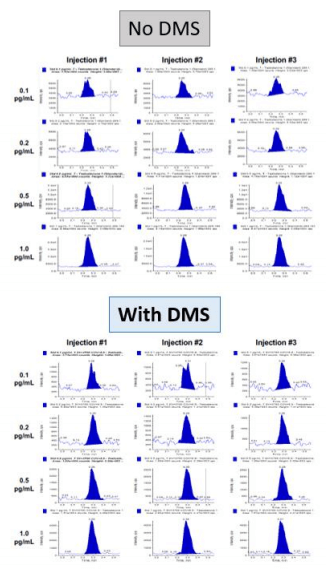 Click to enlarge
Click to enlarge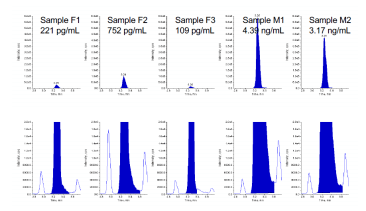 Click to enlarge
Click to enlarge