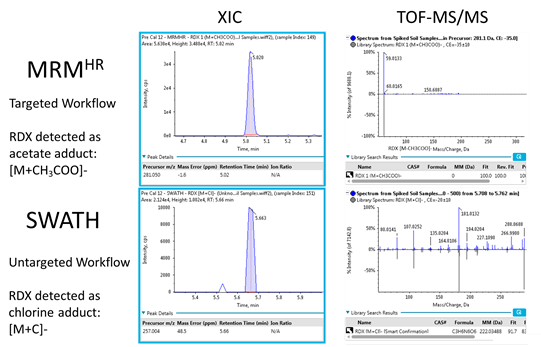
Abstract
Explosive materials are used in many different applications and are finding their way into the environment. To gain insights on the prevalence and impact of these contaminants, sensitive and robust quantitative assays are needed. Here, a fast and simple sample preparation procedure was developed that provide high compound recovery and samples were analyzed using the SCIEX X500R QTOF system. Both a targeted MRMHR workflow and a non-targeted SWATH acquisition workflow were used to acquire high resolution MS and MS/MS spectra on the 12 explosive compounds. The advantages of each method for identification and quantification of explosives and their various adducts is discussed.

Introduction
The widespread use of explosive materials in activities such as military training, blasting, mining, explosive production and other explosive-related applications has led to the their introduction into the environment. Due to their labile nature, explosive materials and residues can migrate through subsurface soil and cause widespread contamination of soil and groundwater. Their ubiquitous presence in the environment is a growing health and public safety concern worldwide due to their toxic and carcinogenic nature. As a result, the ability to rapidly detect and identify trace amounts of explosives in soil is paramount for forensic science, environmental monitoring projects and legal authorities alike. Being able to accurately quantify explosives and their residues in different soil samples is critical to gain insights into the extent of the contamination and to assess the risk associated with their presence in the environment.
The combination of chromatography with tandem mass spectrometry (LC-MS/MS) provides the required levels of sensitivity and specificity for the detection of explosives. Given their intrinsic richness in electron-withdrawing nitrogen-containing groups and their thermally instable nature, explosives are well-suited for analysis by LC-MS/MS. Here, a high-throughput and sensitive detection method for accurate quantification and confident identification of 12 explosives in soil samples, combining a fast and simple sample preparation procedure with analysis on the SCIEX X500R QTOF system, is described.