Analysis of Kratom’s main psychoactive components: mitragynine and 7-hydroxymitragynine
Using Scheduled MRM™ Algorithm on the SCIEX QTRAP® 4500 LC-MS/MS System
Holly McCall1, Casey Burrows1, Leonard Chay2, Xiang He1, and Alexandre Wang1
1SCIEX, 1201 Radio Rd, Redwood City, CA 94065, USA; 2SCIEX, 71 Four Valley Dr, Concord, Ontario, L4K 4V8 Canada
Abstract
Kratom (Mitragyna Speciosa - a plant native to Southeast Asia) has been put onto the U.S. DEA Scheduled I drugs of abuse list, as it has high potential for recreational abuse. In order to effectively identify the two key components of Kratom (mitragynine and its metabolite, 7-hydroxymitragynine (7-HMG)) in urine specimens, forensic laboratories require sensitive and robust MS systems along with dependable chromatography.A quantitative MRM method for LC-MS/MS analysis of kratom compounds was established on the SCIEX ExionLC™ AC HPLC System and the SCIEX QTRAP® / Triple Quad™ 4500 LC-MS/MS System. The dilute-and-shoot sample preparation procedure provided excellent sensitivity and linearity for the identification of these analytes in a human urine specimen.

Introduction
Kratom (Mitragyna Speciosa) is a plant native to Southeast Asia that is typically consumed as a pill, capsule or extract.1 Two components in kratom leaves, mitragynine and its metabolite, 7-hydroxymitragynine (7-HMG), are known to interact with the opioid receptors in the brain. These interactions cause the user to feel sedation, pleasure, and decreased pain. While mitragynine is the primary constituent of the plant material, 7-HMG has a greater affinity for the opioid receptors making it more potent than mitragynine itself.
Finding its way into the recreational drug market, kratom is believed to have a high potential for abuse. Due to this concern, the United States Drug Enforcement Agency (U.S. DEA) attempted to list kratom as a Schedule I drug of abuse as early as 2016.2 Yet, they have consistently been met with protests. At this time, no federal regulation on kratom consumption exists for the U.S. and many European countries. Australia and some Southeast Asian countries have banned the possession and use of kratom, and others have outlawed its use in traditional medicines and health supplements.
In order to effectively identify these active alkaloids in urine specimens, forensic laboratories require sensitive and robust MS systems along with dependable chromatography. In this method, quantification of mitragynine and 7-HMG in human urine matrix is demonstrated from 2 – 500 ng/mL.
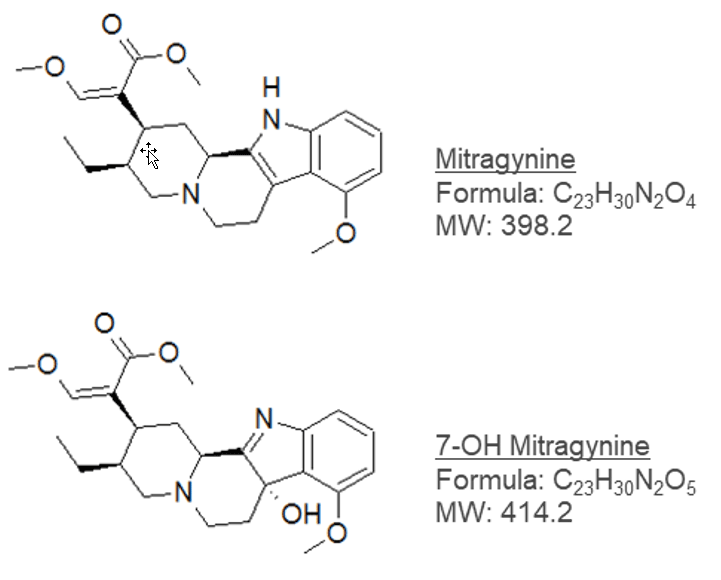
Figure 1: Structures of mitragynine and 7-OH mitragynine.
Key features of the QTRAP® 4500 LC-MS/MS System
- The SCIEX QTRAP 4500 System is designed to deliver a high level of sensitivity and robustness even for the most complex matrices, making it an ideal fit for forensic screening and confirmation applications
- The Turbo V™ Source with Curtain Gas™ Interface reduces chemical noise and allows the use of up to 5 mL/min flow rates
- QJet® Ion Guide captures and focuses ions into the high vacuum chamber using a combination of gas dynamics and RF fields; capturing more of the free jet expansion and significantly increases overall sensitivity over skimmer designs
- Curved LINAC® Collision Cell permits greatly reduced pause and dwell times without a loss in sensitivity, allowing multi-target analyses
- Unique QTRAP System technology offers up to 100X more full-scan sensitivity over basic triple quadrupole MS system
- Ultra-fast scan speeds improve precursor ion and neutral loss scan performance allowing the duty cycle to match the time scale required by fast LC
- Scheduled MRM™ Algorithm maximizes dwell time without sacrificing quantitative accuracy and precision
Experimental details
Stock preparation: Stock solutions of analytes and internal standards were prepared separately. A 5,000 ng/mL stock solution of mitragynine and 7-HMG was created in MeOH. A series of dilutions in MeOH, followed by subsequent dilutions in human urine resulted in the working concentrations for the calibrators (2, 5, 10, 20, 100, and 500 ng/mL) and QCs (10 and 200 ng/mL).
Internal standards: Mitragynine-D3 and 7-hydroxymitragynine-D3 were purchased from Cerilliant and used as internal standards. The two were combined and diluted in methanol to a resulting concentration of 2 µg/mL.
Sample preparation: The samples were prepared according to the following procedure:
- 100 µL of urine sample, 25 µL of hydrolysis buffer were added to each sample and vortexed. 20 µL of recombinant β-Glucuronidase solution was then added and vortexed.
- 50 µL of IS spiking mix was added to all samples, except for the double blank where water was spiked.
- Samples were incubated for 30 minutes at 55°C to complete hydrolysis then centrifuged.
- 75 µL of supernatant was transferred to an autosampler vial
- 325 µL of sample diluent was added to all samples
- Samples were vortexed, and transferred to the autosampler for analysis by LC-MS/MS.
HPLC Conditions: HPLC separation was achieved using a Phenomenex Biphenyl column (50 × 3 mm, 2.6µm, 00B-4462-Y0) with a Phenomenex SecurityGuard™ ULTRA UHPLC Biphenyl (AJ0-9207) and ULTRA holder (AJ0-9000) on the SCIEX ExionLC™ AC HPLC System. Mobile phase A (MPA) and mobile phase B (MPB) were formic acid in water and formic acid in a mixture of methanol and acetonitrile. The injection volume was 10 µL and the total LC run time was 4.5 min.
Mass Spectrometry: Data was collected using positive electrospray ionization (+ESI) on the SCIEX QTRAP 4500 LC-MS/MS System with Analyst® Software 1.7. The Scheduled MRM Algorithm was used in order to acquire an adequate amount of data points for quantifiable data.
Figure 2: Chromatogram showing the elution profile of analytes. Using targeted MRM detection, mitragynine and 7-HMG were detected at 2.22 and 1.96 min, respectively, when using the optimized LC method.
Chromatographic separation of mitragynine and 7-hydroxymitragynine
Baseline resolution for mitragynine and 7-hydroxymitragynine was achieved using the ExionLC AC System with the Biphenyl column. Figure 2 shows the elution profile of mitragynine and 7-HMG, which eluted at 2.23 and 1.96 min, respectively. The stability of the LC method was evaluated by repeat analysis of the calibration standard for over 70 injections. As shown in Figure 3, minimal variability in retention time (RT) was observed over the course of the repeated injections.
Figure 3: Retention time reproducibility of method. Metric plot of retention time across 70 injections highlights the high chromatographic reproducibility of the method.
Figure 4: Extracted ion chromatograms (XICs) of the quantifier MRM of mitragynine (n=3). Replicates are shown across the following range of concentrations (A) 2 ng/mL; (B) 5 ng/mL; (C) 10 ng/mL; (D) 20 ng/mL; (E) 100 ng/mL; (F) 500 ng/mL.
Figure 5: Extracted ion chromatograms (XICs) of the quantifier MRM of 7-HMG (n=3). Replicates are shown across the following range of concentrations (A) 2 ng/mL; (B) 5 ng/mL; (C) 10 ng/mL; (D) 20 ng/mL; (E) 100 ng/mL; (F) 500 ng/mL. |
Analytical performance of the QTRAP 4500 System
The urine samples underwent a 10-fold dilution during sample preparation, and both compounds were detected with excellent sensitivity. Figures 4 and 5 show the analysis of mitragynine and 7-HMG at the each of the different calibration levels evaluated over three replicates. It can be observed that, even at the lowest level of quantitation (LLOQ) of 2 ng/mL, a strong peak is present with a signal-to-noise ratio greater than 10. These signals suggest that the limits of detection (LOD) have the potential to be lower. Calibration curves for both analytes produced good linearity with correlation (R2) of greater than 0.99 across the 2.5 orders of concentration studied.
Precision and accuracy were established using five spiked matrix samples at two levels (10 and 200 ng/mL). Three replicates were analyzed at each of these levels. Acceptance criteria of ≤ 20% CV (coefficient of variation) for precision and accuracy of ± 20% was used for the analysis. All samples yielded acceptable results.
Carryover was evaluated by injection of a blank following a sample containing 500 ng/mL (ULOQ) of each analyte. No carryover was observed for either analyte in the method at that concentration. Samples with concentration above the ULOQ should be diluted and re-analyzed to ensure measurement within the linear dynamic range of the assay.
Conclusions
A quantitative MRM method for LC-MS/MS analysis of kratom compounds, mitragynine and 7-HMG, was established using the ExionLC AC HPLC System and the QTRAP 4500 System. The dilute-and-shoot sample preparation procedure provided excellent sensitivity and linearity for the identification of these analytes in a human urine specimen.
- A simple dilute-and-shoot protocol was easily implemented to extract the analytes from human urine samples
- A targeted confirmation method using MRMs was developed for the selective detection of kratom compounds, mitragynine and 7-HMG
- The method showed good sensitivity down to the 2 ng/mL level in urine samples
- Acceptable precision and accuracy were achieved at each concentration evaluated when five spiked urine samples were evaluated
References
- What is Kratom? NIDA. 2019, April 8. Kratom DrugFacts.
- DEA Announces intent to schedule Kratom. DEA August 30, 2016.
 Click to enlarge
Click to enlarge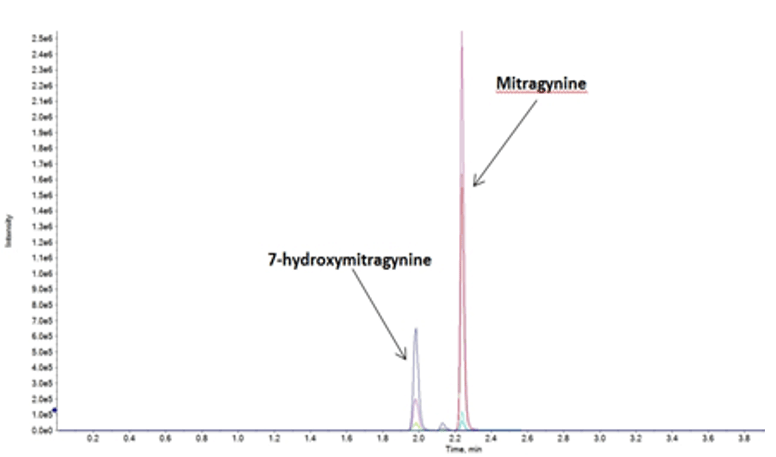 Click to enlarge
Click to enlarge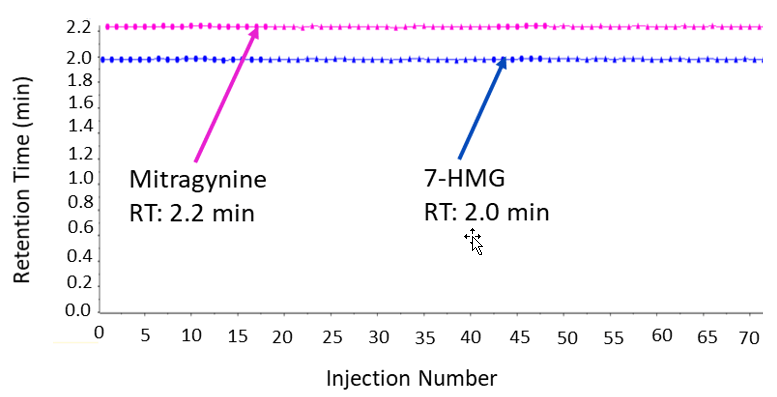 Click to enlarge
Click to enlarge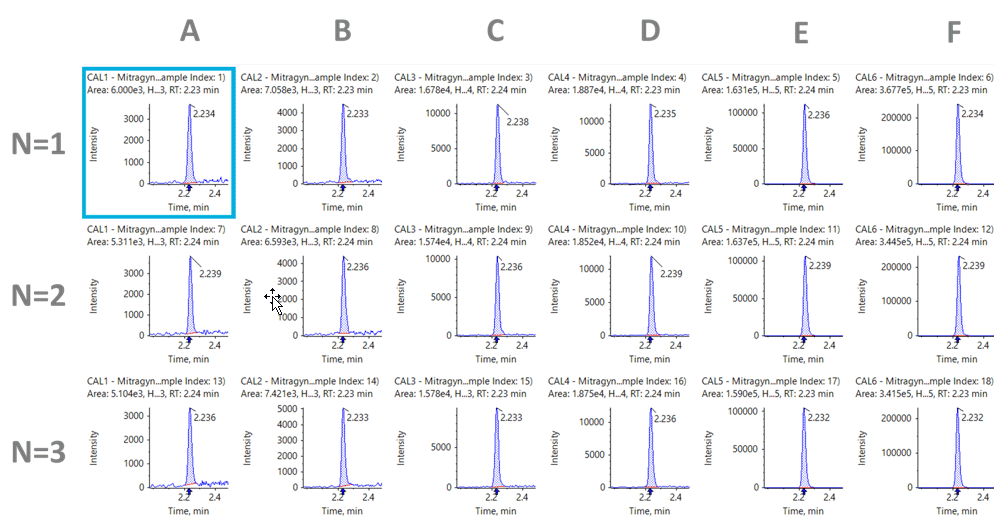 Click to enlarge
Click to enlarge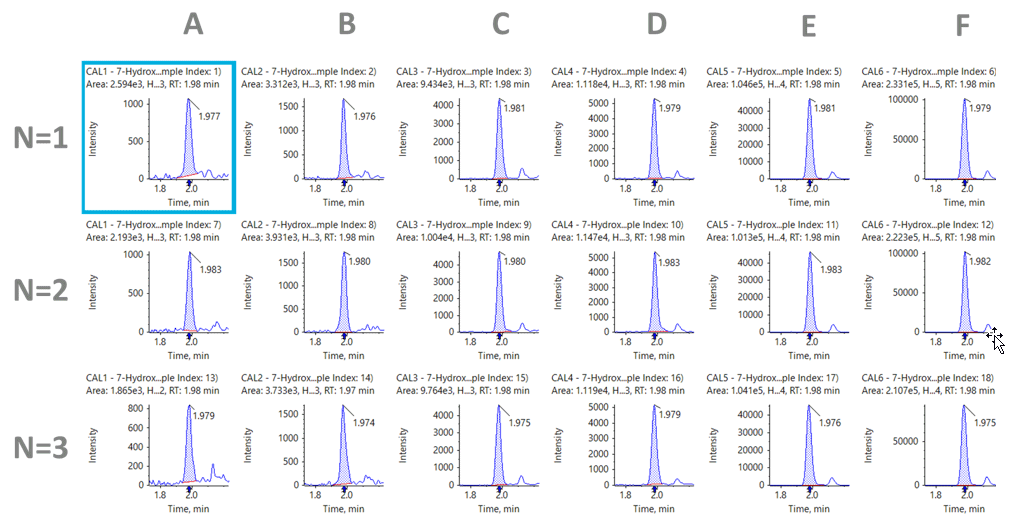 Click to enlarge
Click to enlarge