Determination of novel psychoactive substances (NPS) and synthetic opioids in meconium
Using SWATH acquisition on the SCIEX X500R QTOF system
Ángela López-Rabuñal1, Daniele Di Corcia2, Eleonora Amante2, Marta Massano2,3, Angelines Cruz-Landeira1, Ana de-Castro-Ríos1, Marco Vincenti2,3, Alberto Salomone2,3 and Pierre Negri4
1Servizo de Toxicoloxía, Instituto de Ciencias Forenses, Facultade de Medicina, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2Centro Regionale Antidoping e di Tossicologia “A. Bertinaria”, Orbassano, Turin, Italy, 3Dipartimento di Chimica, Università degli Studi di Torino, Turin, Italy; 4SCIEX, USA
Abstract
A quantitative screening workflow for the detection of 137 NPS combining the use of SWATH acquisition on the SCIEX X500R QTOF system with a solid phase extraction (SPE)-based sample preparation method. This robust and quantitative screening workflows enabled confident identification and accurate quantification of NPS present in 30 meconium specimens from cases in which fentanyl had been administered as epidural anesthesia at the time of delivery or cases in which the maternal hair was positive to other drug of abuse.

Introduction
Novel psychoactive substances encompass a class of drugs that are newly synthetized or newly available and which do not fall under the control of the United Nations Office on Drugs and Crime (UNODOC). As such, they are defined as “substances of abuse, either in a pure form or a preparation, that are not controlled by the 1961 Single Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances, but which may pose a public health threat”.1 The widespread emergence of these designer drugs on the recreational drug market constitutes a public safety threat worldwide, as these substances vary greatly in purity and potency.
Of the various populations exposed to NPS, pregnant women are among the most vulnerable to the harmful effects of these substances. Prenatal or in utero NPS exposure presents considerable health risks for both the carrying mother and the developing fetus and has been associated with conditions such as neonatal abstinence syndrome (NAS), birth defects, low birth weight, preterm delivery and other long-term neurodevelopmental disorders that affect brain structure and function.2-5 Meconium, which is a newborn’s first stool, is considered the best matrix for detecting prenatal drug exposure. Although complex in composition, meconium provides a wide detection window that covers the second and third trimesters of pregnancy.6-7 Therefore, reliable screening methods capable of identifying and quantifying drug use in meconium samples provide unequivocal evidence of prenatal exposure to NPS and synthetic opioids.
In this technical note, a quantitative screening workflow for the detection of 137 compounds in meconium is described, combining the use of SWATH acquisition on the SCIEX X500R QTOF system with a solid phase extraction (SPE)-based sample preparation method. This robust and quantitative screening workflow enabled confident identification and accurate quantification of compounds in 30 authentic meconium specimens from cases in which fentanyl had been administered as epidural anesthesia at the time of delivery or cases in which maternal hair tested positive for other drugs of abuse.
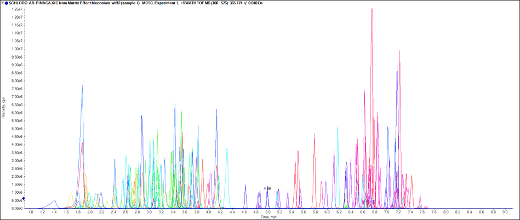
Figure 1. Rapid detection of the 137 NPS extracted from meconium. Chromatographic profile showing the extracted ion chromatogram (XIC) resulting from the optimized LC conditions using a spiked meconium calibrator solution containing the 137 molecules targeted in this study.
Key features of prenatal NPS and synthetic opioids screening in meconium
- Solid phase extraction (SPE) for selective extraction of drugs from meconium samples
- SWATH acquisition for untargeted data acquisition, robust detection of analytes in biological samples and reproducible quantification with sub-ng/g detection of NPS
- Flexible data analysis pipeline allows users to add new NPS and synthetic opioids to the spectral library, permitting retrospective analysis of previously acquired samples.
Experimental details
Target analytes and solutions: A total of 137 molecules including 54 synthetic cannabinoids and metabolites, 49 synthetic cathinones, stimulants, dissociatives and hallucinogens, 34 fentanyl analogs and synthetic opioids as well as 13 deuterated internal standards were purchased from Cerilliant Corporation (Round Rock, TX). Two solutions were prepared in water: a standard solution containing the 137 molecules and an internal standard solution containing the 13 deuterated standards. Table 1 lists the class and name of the analytes included in the panel.
Meconium samples: Blank meconium specimens used for the calibrator solutions were collected at the University Hospital of Vigo (Galicia, Spain) from newborns whose mothers were not suspected of drug use during pregnancy. Meconium was collected at the hospital from diapers of newborns up to 3 days after delivery and stored in polypropylene containers at -20 °C until analysis. Thirty authentic meconium specimens were collected at the University Hospitals of Santiago de Compostela and Vigo (Galicia, Spain) from January 2012 to December 2015.
Calibrator preparation: Calibrators were prepared by spiking the standard solution containing the 137 target analytes in blank meconium samples. Eight concentrations were tested, ranging from 2 to 1000 ng/g.
Sample homogenization and solid phase extraction (SPE) procedure: Blank and authentic meconium specimens spiked with various concentrations of the 137 molecules were homogenized and subjected to SPE using the sample extraction procedure summarized in Figure 2.
Figure 2. Meconium specimen homogenization and solid phase extraction (SPE) workflow. A 14-step sample extraction protocol was optimized to selectively extract the 137 molecules from meconium for analysis using the SCIEX X500R QTOF system. Note that 50 µL of a 1% HCl solution in methanol was added to prevent analyte evaporation before each evaporation step.
Liquid chromatography: UHPLC separation was performed on a Phenomenex C18 column (100 x 2.1 mm, 1.7 µm, 00D-4475-AN) at 45 °C on the ExionLC AC system. Mobile phases consisted of water, acetonitrile and modifiers. The LC flow rate was 0.5 mL/min and the total run time was 10 min. The injection volume was 3 µL.
Mass spectrometry: MS and MS/MS data were collected for each sample using SWATH acquisition on the SCIEX X500R QTOF system in positive mode. Data acquisition was TOF MS scan followed by 12 MS/MS scans using variably sized Q1 windows, covering a mass range from 150 to 465 Da. Data were acquired using SCIEX OS Software 1.5.
Data analysis: Data processing was performed using SCIEX OS software 1.5.
Developing a robust screening method for NPS detection
Blank meconium samples were spiked with the stock standard solution in concentrations ranging from 2 to 1000 ng/g. The NPS and synthetic opioids were extracted from the meconium samples using the aforementioned procedures, consisting of a homogenization step followed by an SPE-based extraction method. The extracted samples were injected in triplicate on 6 consecutive days to build a data processing method. Figure 1 displays the extracted ion chromatogram (XIC) resulting from the chromatographic separation of the 137 molecules using a blank meconium sample spiked at 50 ng/g with the stock standard solution.
Selective extraction procedure results in minimal matrix effects and absence of interference
Meconium is a complex biological matrix due to its heterogegenous composition, which often causes ion suppression or detection interference of target analytes when using LC-MS. The efficiency of the sample preparation procedure to selectively remove the matrix interferences was determined by calculating the matrix effect (ME). ME is defined as the ion suppression/enhancement ratio and is expressed as ±%. The average ME (±%, n=3) was calculated by expressing the ratio of the average peak area of each analyte in neat solvent vs. post-extraction spiked matrix as a percentage, at low (50 ng/g) and high (1000 ng/g) concentration levels. The ME ranged from -70 to 72% for synthetic cannabinoids, -89 to 71% for synthetic cathinones and hallucinogens and -88 to 110% for fentanyl-analogous and synthetic opioids. The ME values at low and high concentrations are summarized in Table 1.
The selectivity and specificity of the method was verified for each of the 137 NPS molecules included in the panel. Neither endogenous nor exogenous interferences with a signal/noise ratio above 3 were detected near the retention times of the analytes. To test whether there was carry-over between injections, blank samples were injected after the higher level of the calibration curve. No relevant signal was detected for the blank samples, confirming the absence of carry-over between injections.
SWATH acquisition provides sensitive detection of analytes in meconium
Reliable quantification of drugs and metabolites extracted from meconium is key to accurately determine the levels of prenatal drug exposure. The use of a robust detection method is critical to achieve reproducible and accurate determination of drug concentrations. In this study, SWATH acquisition was used to simultaneously perform quantification and confirm identification of the 137 molecules by using the precursor ions for quantification and the MS/MS spectra for accurate analyte identification through spectral library matching. The ability to accurately and reproducibly measure various analyte levels in extracted meconium using SWATH acquisition was investigated.
Figure 3 shows representative XICs for 3 drugs representative of each of the 3 NPS classes included in this panel. XICs are shown for A) JWH-147, a synthetic cannabinoid, B) ethylone, a stimulant and C) carfentayl, a fentanyl analog. The XIC traces display the signal for a blank injection (left) and for 8 concentrations ranging from 2 to 1000 ng/g. The lower limit of detection (LLOD) for the 137 NPS molecules targeted in this study ranged from 0.04 to 2.4 ng/g.
Calibration curves were generated for each of the 137 molecules. Figure 4 shows representative regression lines from each of the 3 NPS classes, plotted from 2 to 1000 ng/g. The calibration curves showed excellent linear responses across the calibration series, with R2 values greater than 0.98 for all the NPS targeted in the panel. These parameters demonstrate the broad applicability of the sample preparation procedure to a variety of NPS classes.
Figure 3. Representative extracted ion chromatograms (XICs) for each of the drug classes included in the panel. XIC traces for A) the synthetic cannabinoid, JWH-147, B) the synthetic cathinone, ethylone and C) the fentanyl analog, carfentanyl from the series of 8 calibrator solutions ranging from 2 to 1000 ng/g.
Figure 4. High correlation demonstrated for the 137 molecules included in the panel. Calibration curves resulting from the calibration series for A) 54 synthetic cannabinoids, B) 49 synthetic cathinones, dissociatives and hallucinogens and C) 34 fentanyl analogs and synthetic opioids, across 3 orders of linear dynamic range. Excellent correlation was observed with R2 values greater than 0.98 for all the compounds included in this panel.
Reproducibility of NPS quantification
The ability to reproducibly quantify drugs and metabolites extracted from meconium samples was investigated by performing 6 consecutive injections and calculating the average %CV value for each of the 137 molecules. These %CV values were consistently below 20% (Table 1), indicating that this screening workflow using the SCIEX X500R QTOF system is capable of precise quantification.
Accurate identification and quantification in authentic meconium specimens
The robustness and quantitative performance of the screening workflow was further investigated by analyzing 30 authentic meconium specimens from newborns whose mothers were administered fentanyl through epidural anesthesia at the time of delivery (N=26) or from cases in which maternal hair tested positive for other drugs of abuse (N=4) after delivery. These authentic samples were prepared using the aforementioned extraction method and run using the developed screening method.
Four meconium specimens tested positive for fentanyl at concentrations ranging from 440 to 750 ng/g and 2 specimens tested positive to acetylfentanyl at concentrations ranging from 190 to 1400 ng/g. A few observations can be drawn from the results of the authentic meconium specimens analysis. First, 2 of the fentanyl-positive specimens were cases in which fentanyl was administered as epidural anesthesia during labor. The third fentanyl-positive specimen was a case in which fentanyl was administered during labor and also detected in the mother's hair throughout the course of pregnancy (5.0, 5.7 and 4.9 pg/mg for the first, second and third trimester, respectively) using a validated method.8 These data might suggest that fentanyl was ingested illegally during the course of pregnancy.
The fourth fentanyl-positive specimen was a case in which fentanyl was not found in the maternal hair. Information regarding the use of fentanyl during epidural anesthesia was not available. These data suggest either administration of fentanyl during labor or illicit intake during pregnancy. The maternal hair tested positive for MDMA in the first trimester of pregnancy at a concentration of 162 pg/mg, suggesting the mother was a chronic drug user.
Table 2 summarizes the results of the 4 positive meconium specimens along with the information regarding the epidural anesthesia and the results of the maternal hair testing. The results of the analysis of the 30 authentic meconium specimens demonstrate the robustness of the screening workflow and its ability to reliably quantify various levels of NPS and synthetic opioids.
Table 2. Summary of the 4 fentanyl-positive authentic meconium specimens.
Conclusions
A quantitative screening workflow was successfully developed for the detection of 137 molecules, including synthetic cannabinoids, synthetic cathinones, dissociatives, hallucinogens, fentanyl analogs and synthetic opioids, as well as some metabolies extracted from meconium. The combination of a selective sample preparation method, including homogenization followed by solid phase extraction (SPE), and the use of SWATH acquisition on the SCIEX X500R QTOF system enabled robust quantification of drugs and metabolites with a wide range of physical and chemical properties.
- Excellent precision with %CV values below 30% for all 137 molecules extracted from meconium samples on 6 consecutive days
- Excellent correlation (R2 >0.98) across 3 orders of linear dynamic range, from 2 to 1000 ng/g, for all analytes included in the panel
- Detection limits for the analytes included in the panel ranged from 0.04 to 2.4 ng/g
- Matrix effect ranged from -70 to 110% for all the molecules included in the panel, demonstrating the robustness of the sample preparation method for selectively extracting the drugs and metabolites from meconium samples
This method was then used to analyze 30 authentic meconium specimens. Four meconium specimens tested positive for fentanyl at concentrations ranging from 440 to 750 ng/g, and 2 specimens tested positive for acetylfentanyl at concentrations ranging from 190 to 1400 ng/g. The results provided evidence and a quantitative measure of prenatal NPS and synthetic opioid exposure.
The data-independent nature of the SWATH acquisition used for data collection enables retrospective analysis of previously acquired data. In cases in which further investigation is required, this enables screening for compounds discovered since the time of analysis, without requiring samples to be re-analyzed. In addition, as new substances emerge on the recreational drug market, this quantitative screening workflow could easily be updated to include a larger number of NPS and synthetic opioids.
References
- UNODC. UNODC Early Warning Advisory (EWA) on New Psychoactive Substances (NPS).
- Chomchai S, Phuditshinnapatra J, Mekavuthikul P, Chomchai C. (2019) Effects of unconventional recreational drug use in pregnancy. Seminars in Fetal and Neonatal Medicine. 24(2): 142-148.
- Kuczkowski KM. (2013) Anesthetic implications of drug abuse in pregnancy. J. Clin. Anesth. 15(5): 382-394.
- Smid MC, Metz TD, Gordon AJ. (2019) Stimulant use in pregnancy: an under-recognized epidemic among pregnant women. Clin. Obstet. Gynecol. 62(1): 168-184.
- Ross EJ, Graham DL, Money KM, Stanwood GD. (2015) Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 40(1): 61–87.
- Concheiro-Guisan A, Concheiro M. (2014) Bioanalysis during pregnancy: recent advances and novel sampling strategies. Bioanalysis. 6(23): 3133-3153.
- Concheiro M, Huestis MA. (2018) Drug exposure during pregnancy: analytical methods and toxicological findings. Bioanalysis. 10(8): 587-606.
- Lendoiro E, Quintela O, de Castro A, Cruz A, López-Rivadulla M, Concheiro M. Target screening and confirmation of 35 licit and illicit drugs and metabolites in hair by LC–MSMS. Forensic Sci Int. 217(1–3): 207–215.
Table 1. Statistical results for the 137 molecules targeted in this workflow. The table includes the class and name of each compound, LOD and calibration range, correlation coefficient (R2) value, averaged (N=6) inter-day precision (%CV) as well as the averaged (N=5) matrix effect (±%) at low (50 ng/g) and high (1000 ng/g) concentrations for the 137 molecules included in this study.
Table 1. Statistical results for the 137 molecules targeted in this workflow. Continued
Table 1. Statistical results for the 137 molecules targeted in this workflow. Continued
Table 1. Statistical results for the 137 molecules targeted in this workflow. Continued
Table 1. Statistical results for the 137 molecules targeted in this workflow. Continued
 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge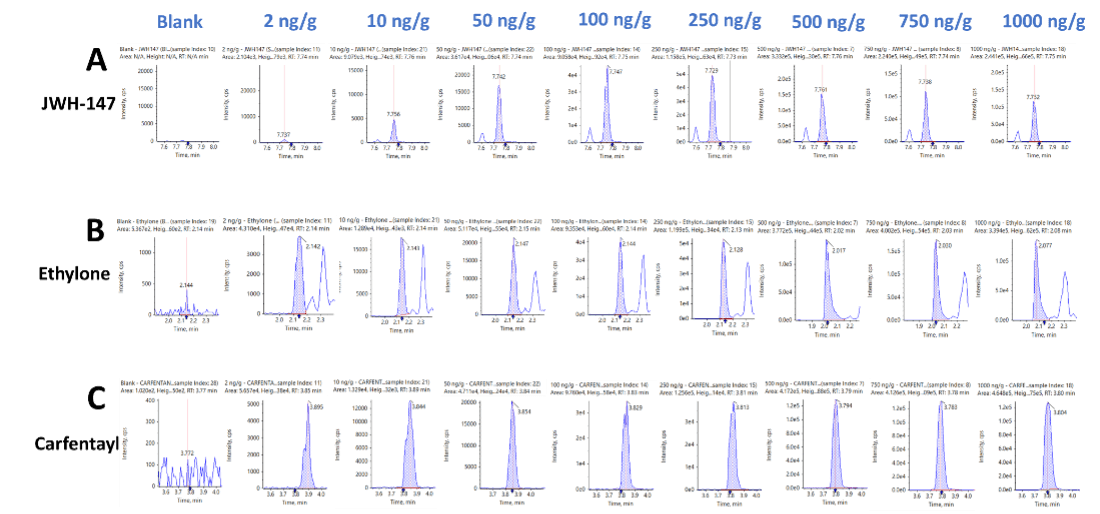 Click to enlarge
Click to enlarge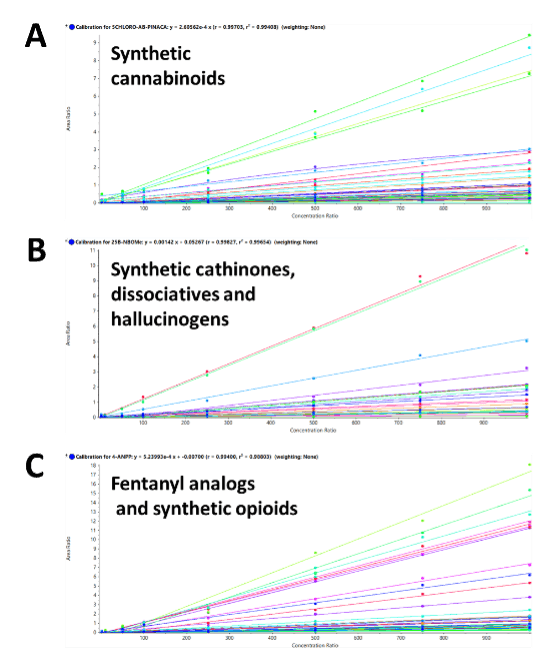 Click to enlarge
Click to enlarge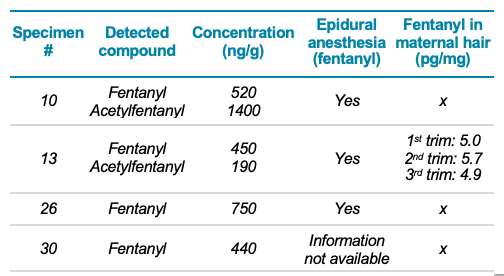 Click to enlarge
Click to enlarge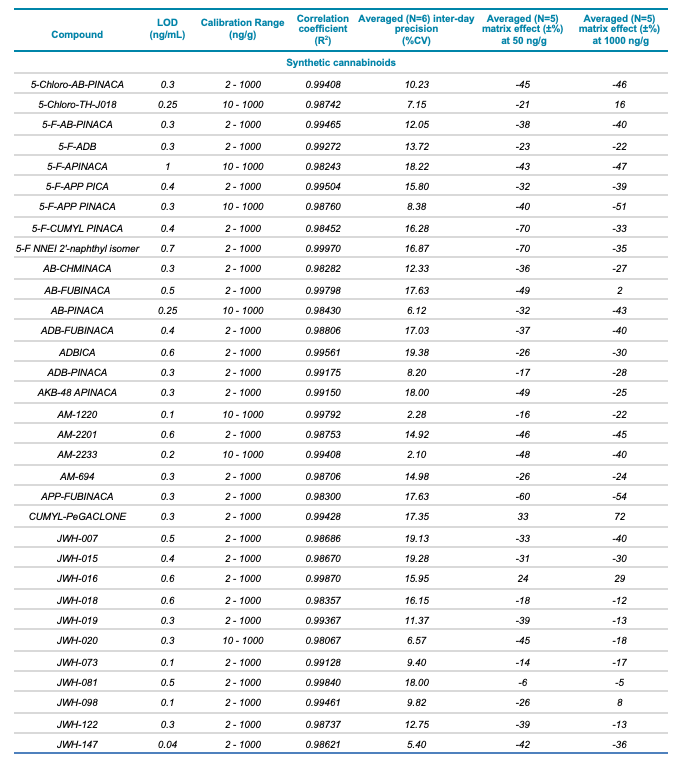 Click to enlarge
Click to enlarge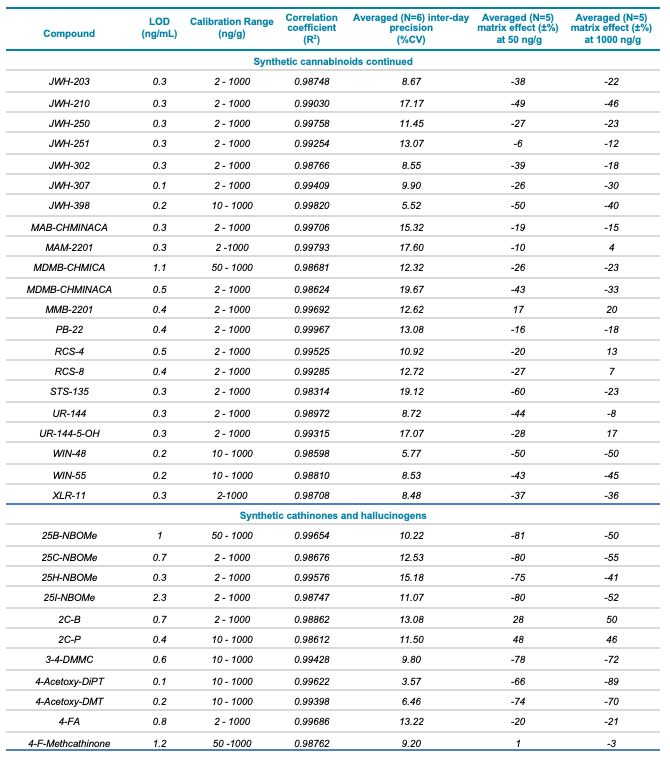 Click to enlarge
Click to enlarge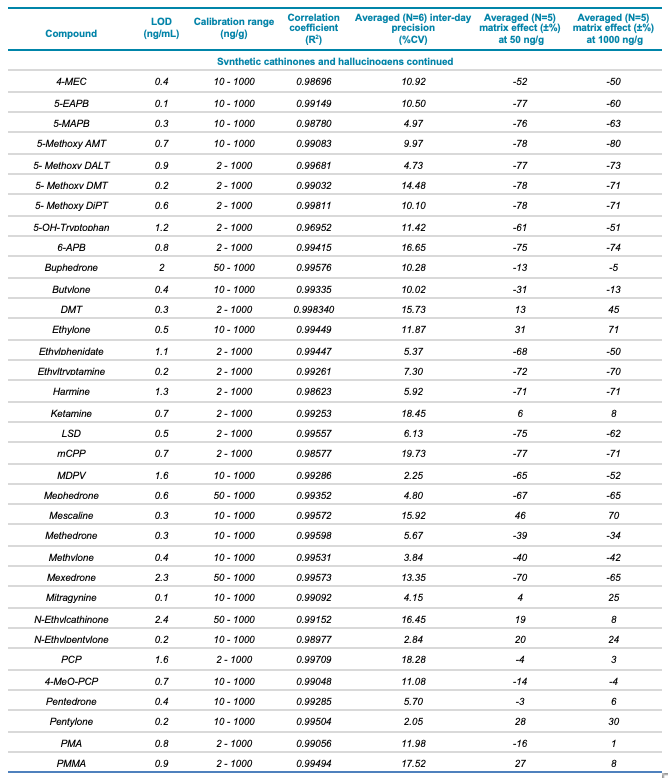 Click to enlarge
Click to enlarge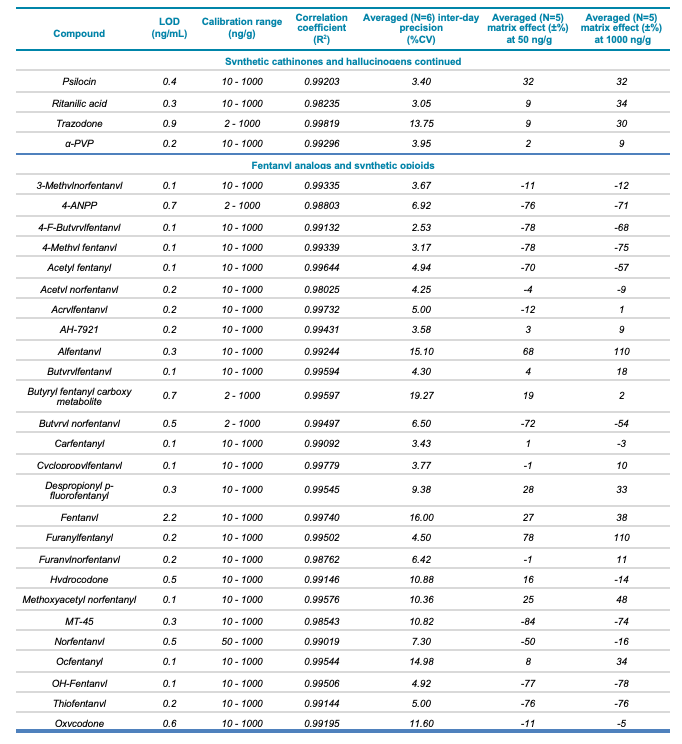 Click to enlarge
Click to enlarge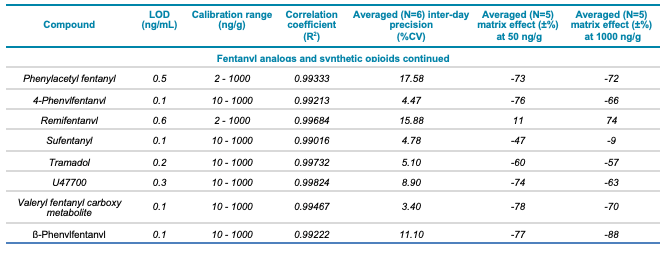 Click to enlarge
Click to enlarge