Detection of fentanyl analogs and novel synthetic opioids in hair
Using SWATH® Acquisition on the SCIEX X500R QTOF System
Pierre Negri1, Daniele Dicorcia2, and Alberto Salomone2
1SCIEX, USA; 2Centro Regionale Antidoping e di Tossicologia “A. Bertinaria”, Orbassano, Turin, Italy
Introduction
The recent outburst of novel synthetic opioids (NSO) into the recreational drug market has been a major contributor to the ongoing opioid crisis. These substances are gaining popularity as substitutes to controlled opioids and are often used as cutting agents or adulterants to heroin and other commonly abused drugs. Since the composition and potency of these drugs is highly variable, their use can lead to severe intoxication and overdose fatalities. As a result, the widespread availability of these substances continues to pose major safety concerns for public health and law enforcement officials alike.
Although originally developed by pharmaceutical companies to mimic the effects of traditional prescribed opioids, these substances are not well studied in humans. As a result, timely, and comprehensive drug screening approaches are critically needed to enable forensic laboratories to rapidly and accurately identify these emerging novel substances. Detection of these drugs can be performed in many biological matrices, such as urine, blood, saliva and hair. Among these matrices, hair is becoming extremely valuable in determining the long-term use of these substances. In addition to the non invasive nature of sample collection, storing hair samples only requires wrapping the collected hair segments in aluminum foil and keeping them under dry conditions in the dark at room temperature to minimize the risk of sample degradation overtime. The keratin matrix in hair incorporates the parent NPS consumed by the subject, offering a much wider diagnostic window than urine, blood or saliva. These attributes enable forensic researchers to obtain significant retrospective information about past exposure and long-term use of NSO in the consumer’s population.
In this technical note, a comprehensive workflow is presented combining the use of the SCIEX X500R QTOF System with a simple extraction procedure for specific and sensitive detection of fentanyl analogs and synthetic opioids in hair.
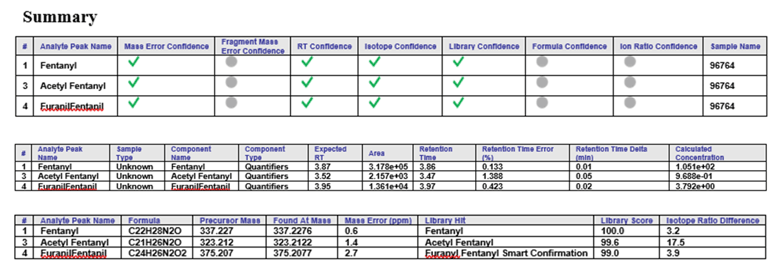
Figure 1: Confidently identify and quantify all NSO present in a head hair sample. Results table showing all positively identified NSO in a real head hair sample. The report showing detailed sample results and comprehensive information on the detected NSO based on the different confidence criteria is easily generated using SCIEX OS Software.
Key features of SWATH Acquisition method for NSO detection in hair samples
- Simplified extraction procedure provided an easily implemented detection method for selective and sensitive trace analysis of fentanyl analogs and synthetic opioids in hair
- SWATH Acquisition provided selective MS/MS detection of all detectable compounds extracted from biological samples, creating a digital record of the sample
- Acquisition method generated comprehensive and high quality MS/MS spectra, enabling reliable compound fragmentation comparison to library spectra for confident NSO identification
- The workflow showed excellent accuracy and precision, with linearity resulting in R2 values >0.99 for all analytes
- Method was applied to real hair samples collected from subjects who had reported past-year non-medical opioid and/or heroin use
- Method allowed identification and quantification of sub pg/mg detection limits of NSO and their metabolites in these real head hair samples
- New analytes can be added to the analytical panel at any time without changing acquisition method and re-interrogation of the sample is possible should new questions or compounds arise in the future
Experimental details
Hair sample preparation and digestion: ~50 mg of head hair were twice-washed with dichloromethane and then methanol (1 mL of solvent, vortex mixed for 3 minutes). The solvent washes were removed following each vortex mixing steps. Following the washing steps, the hair was dried at room temperature using a gentle nitrogen flow and subsequently grinded with a ball mill. The resulting hair samples were spiked with 2.5 µL of an internal standard mixture yielding a final concentration of 50 pg/mg. 1 mL of HPLC grade methanol was added to the hair mixture and the samples were incubated at 55˚C for 15 hours without stirring. Following the incubation step, the organic phase was collected in a UHPLC sample vial. A summary of the head hair samples preparation and extraction procedure is shown in Figure 2. The full list of the compounds and internal standards used in this method is detailed in Table 1.
Calibrator Preparation: A 1 µg/mL stock standard solution mixture, containing all the NSO used to develop the method in this study, was prepared via dilution of 1mg/mL stock standards with methanol. A series of six calibrator solutions were prepared using the 1 µg/mL stock standard solution mixture to evaluate the dynamic range. These calibrator solutions included the following concentrations: 2, 5, 10, 25, 50 and 100 pg/mg. These, in turn, were used to spike ~25 mg of blank head hair samples. Finally, 2.5 µL of an internal standard mixture was added to each hair mixture, resulting in a final concentration of internal standard of 50 pg/mg.
Figure 2. Hair samples preparation and extraction workflow. A 12-step extraction protocol was used for selectively extracting the target analytes and internal standards from hair samples for analysis with the SCIEX X500R QTOF System.
Liquid chromatography: UHPLC separation was performed on a Phenomenex C18 column (100 x 2.1 mm, 1.7 µm, 00D-4475-AN) at 45ºC on the SCIEX ExionLC™ AC System. Mobile phases used consisted of water, acetonitrile and modifiers. The LC flow rate was 0.5 mL/min and the total run time was 11.5 min. The injection volume was 3 µL.
Mass spectrometry: MS and MS/MS data were collected for each sample using SWATH Acquisition on the SCIEX X500R QTOF System. Data acquisition was TOF MS scan followed by 12 variable Q1 windows covering a mass range from 230 to 450 m/z. The resulting cycle time was 0.555 sec. Data was acquired using SCIEX OS Software 1.5.
Data analysis: Data processing was performed using SCIEX OS Software 1.5 for positive analyte identification based on confidence criteria as previously described.1 The four main confidence criteria used include mass error (M), retention time (R), isotope ratio difference (I), and library score (L). Subsequently, a combined score (C) was calculated based on there four confidence categories (MRIL) with custom weightings.
Table 1. List of the 15 target compounds and 3 internal standards used in this study.
Developing a sensitive workflow for accurate mass detection of novel synthetic opioids in head hair samples
Control head hair samples spiked with all 15 target compounds were prepared at various concentrations ranging from 1 to 50 pg/mg. These standard hair mixtures were spiked with the internal standard mixture, extracted using the aforementioned procedure and injected to build a data processing method.
Figure 3 shows the total ion chromatogram (TIC) for the 15 target compounds in a control head hair sample at a concentration of 100 pg/mg. The optimized LC conditions in conjunction with the choice of mobile phase composition and column selection resulted in baseline separation of all analytes, including the closely related fentanyl analog compounds present in the stock standard solution mixture.
A data processing method was developed to review the SWATH Acquisition data in SCIEX OS Software 1.5 Although the data was acquired in a non-targeted fashion, data processing was performed in a targeted way, which means a compound list consisting of the 15 targeted analytes and 3 internal standards was used to perform the targeted analysis. The processing method included the components table listing the chemical formulae (for extracted ion mass calculation or the precursor molecular ion), mass extraction window, and retention time. The components table also included the accurate mass of several unique fragment ion for each compound.
Detection and integration of the peaks from the background was achieved within the viewing window using the MQ4 algorithm. This ease of use enabled streamlined analysis of replicate injections (n=3) which were used to verify the following validation parameters: linearity, LODs, LOQs, intra-assay precision and accuracy. Calibration curves were generated to evaluate the response and quantification performance of the assay. Figure 4 shows the results from the calibration series performed for fentanyl (A) and furanylfentanyl (B). These calibration curves demonstrated excellent correlation of the generated regression curves covering 3 orders of magnitude with R2 values of 0.99965 and 0.99844 for fentanyl and furanylfentanyl, respectively.
Figure 3: Chromatographic profile of the novel synthetic opioid panel by LC-MS analysis. Total Ion Chromatogram (TIC) resulting from near baseline separation of the 15 fentanyl analogs and novel synthetic opioids used in this study. The total LC runtime was 11.5 min. The numbered peaks are assigned as follows: 1. Norfentanyl, 2. Acetylfentanyl, 3. Ocfentanyl, 4. Acrylfentanyl, 5. 4-ANPP, 6. Fentanyl, 7. U-4700, 8. Furanylfentanyl, 9. α-Methylfentanyl, 10. AH-7921, 11. Cyclopropylfentanyl, 12. Carfentanyl, 13. Buryrilfentanyl, 14. 4-Fluoro-Butyrilfentanyl, and 15. MT-45.
Figure 4. Excellent linearity and high dynamic range for novel synthetic opioids. Calibration curves resulting from the calibration series for fentanyl (A) and furanylfentanyl (B). Excellent linear response and sensitivity were observed with R2 values of 0.993331 and 0.99844 for fentanyl and furanilfentanil, respectively.
Table 2 shows the average (n=3) results from the validation study for the 15 target analytes, spiked at three concentration levels (2, 10 and 100 pg/mg) in control head hair samples. Inter-day and intra-day precision (expressed as percent variation coefficient, CV%) and accuracy (expressed as bias%) were found to be below 25% and 20%, respectively. The assay showed great reproducibility for concentrations ranging over three orders of magnitude, proving the robustness of the overall workflow. Moreover, limits of detection in matrix (LOD) were found to be in the sub pg/mg range for most of the target analytes used in this study.
Table 2: Validation study. Average (n=3) Results From the Validation Study Showing Inter-Day and Intra-Day Precision (%CV) and Accuracy (bias%) as Well as the LOD for the 15 Target Analytes Used in This Study.
Full scan MS/MS with SWATH Acquisition leads to accurate identification of novel synthetic opioids in real hair samples
The robustness of the workflow was further investigated by analyzing real head hair samples from subjects who reported any past-year non-medical opioid use. These head hair samples were prepared using the aforementioned extraction procedure. SWATH Acquisition was used as the detection method on the SCIEX X500R QTOF System to generate comprehensive and high-quality MS/MS spectra, which provided comprehensive compound fragmentation. Because the fragment ions are acquired in high resolution, the detected compounds can be accurately identified through extraction of specific accurate mass fragment ions. These fragment ions can in turn be matched for identification through spectra library database searching.The data analysis component of SCIEX OS Software is an integrated software platform that allows for simultaneously quantification and library matching. This processing is performed at the same time and allows for the results to be displayed within one window that includes the XIC, TOF MS and MS/MS spectra of the sample with library search match. In addition, retention time, mass, isotope ratio error, and mass spectral library search score are also calculated automatically and visualized using the “traffic light” display. Figure 5 shows the successful detection of fentanyl, acetyl fentanyl and furanylfentanyl from one of the tested head hair samples at concentration of 105.1, 0.9688, and 3.792 pg/mL pg/mg, respectively. The library fit scores (>99.0%) and the combined scores (>90%) providing excellent confidence for the definitive detection of these NSOs.
Figure 5: SWATH Acquisition leads to accurate identification and quantification of NSOs in real head hair samples. Extracted Ion Chromatograms (XICs), TOF MS and MS/MS spectra showing confident and detailed identification of fentanyl (top), acetyl fentanyl (middle) and furanylfentanyl (bottom) from a real head hair sample. These three NSOs were accurately detected at 105.1 (fentanyl), 0.9688 (acetyl fentanyl) and 3.792 pg/mg (furanylfentanyl) with excellent MS/MS fit value (>99%) and for all three compounds. |
Efficient sample reporting of positively identified novel synthetic opioids
Another great advantage of the use of the SCIEX OS Software is that it provides the ability to streamline the review of the results and efficiently summarize them in a sample report. Compounds positively identified based on the confidence criteria can be filtered by the “traffic lights” and presented in a results table. In addition, the customized sample generation feature of SCIEX OS Software includes detailed information about the detected analytes such as the retention time error, the calculated concentration as well as the library and combined scores calculated based on the numbers assigned for each of the four confidence criteria.
Figure 6 shows a customized report generated by SCIEX OS Software following the processing of a real head hair sample. The customized report lists fentanyl, acetyl fentanyl and N-methyl norfentanyl as the three NSOs positively identified and includes their XICs, TOF MS and MS/MS spectra as well as the library matches. An interesting observation from the analysis of this sample is the detection of the same fragment mass of 188.143 Da for fentanyl and acetyl fentanyl. This is a common occurrence for fentanyl analogs as these structurally-related compounds are known to share same fragment ions. However, the presence of other fragment ions in their MS/MS spectra provided another dimension of specificity that enabled identification of the related analytes through library matching. The high library matching scores (>99%) together with the resulting combined scores (>90%) provided another level of confidence for the accurate identification of the three NSOs in this head hair sample.
Figure 6: Streamlined review of the results through customized sample reports using SCIEX OS Software. A sample report was generated identifying three NSOs detected in a real head hair sample. The customized sample report includes detailed sample information (left) as well as representative XICs, TOF MS and MS/MS spectra of the detected compounds (right).
Conclusions
A comprehensive workflow for the detection of fentanyl analogs and synthetic opioids in hair was successfully developed using the SCIEX X500R QTOF System. The combination of a simple sample extraction procedure and a highly selective MS/MS acquisition method enabled sensitive detection of NSOs in real head hair samples at sub pg/mg detection limits.
- A simplified sample preparation procedure enabled efficient extraction of NSOs and metabolites from the hair matrix
- SWATH Acquisition generated comprehensive high resolution MS/MS spectra of all detectable compounds present in the hair matrix, creating a digital record of the sample. The resulting MS/MS fragments enabled accurate identification of the NSOs through spectral library matching
- uccessful analyte identification was confirmed using a combination of confidence criteria including mass accuracy, RT, % difference in isotope ratio, MS/MS library matching and the associated combined score
- The data analysis component of SCIEX OS Software (Analytics) provided a simplified interface for streamlined data review based on a robust and reliable scoring system and efficient sample report generation
- Newly discovered NSOs can be added to the panel of target analytes to allow retrospective analysis of previously-acquired data to look for the presence of these new substances
- Development of these comprehensive screening methods will provide law enforcement agencies and health professionals a clearer picture of the long term use of these drugs and their evolution in the consumer market as well as consumption trends in specific population
References
- vMethod™ Application – Single-Injection Screening of 664 Forensic Toxicology Compounds on a SCIEX X500R QTOF System.

 Click to enlarge
Click to enlarge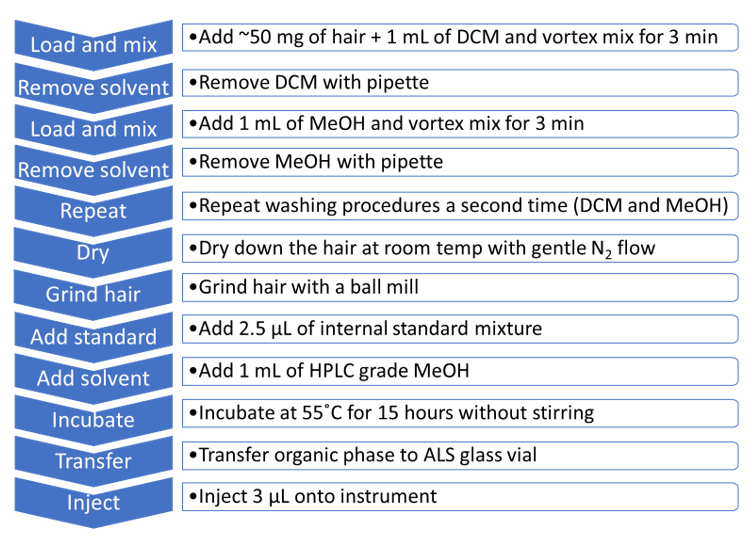 Click to enlarge
Click to enlarge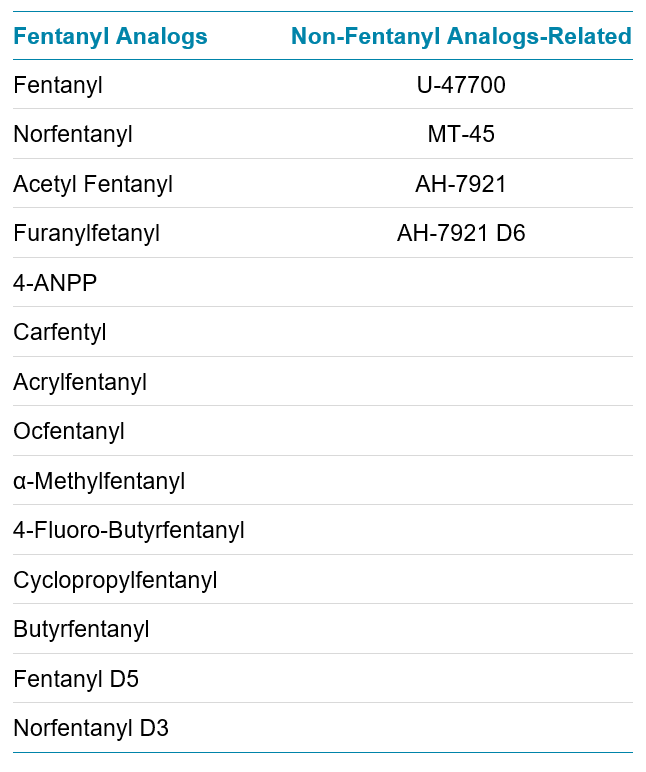 Click to enlarge
Click to enlarge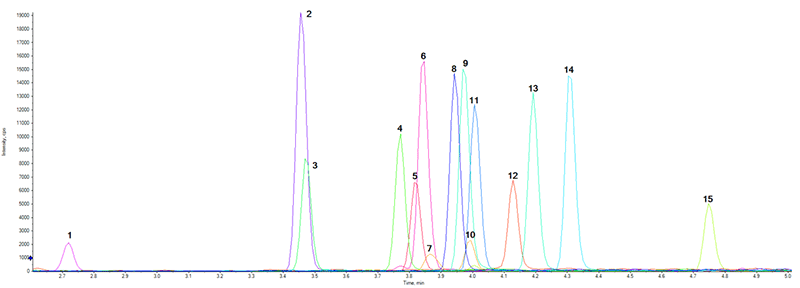 Click to enlarge
Click to enlarge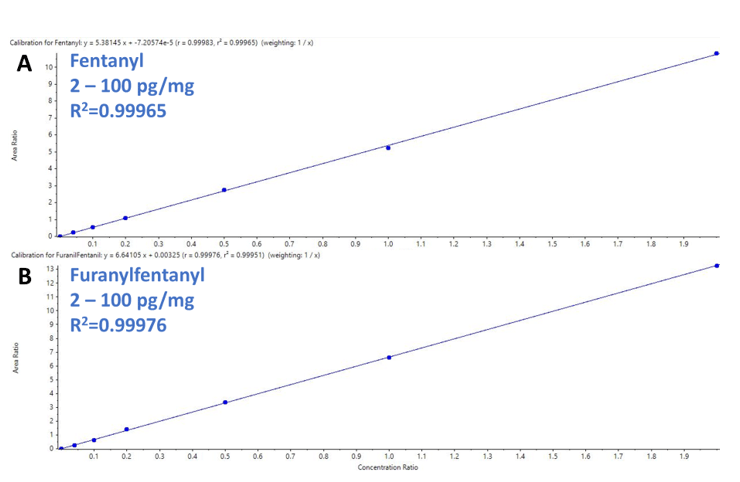 Click to enlarge
Click to enlarge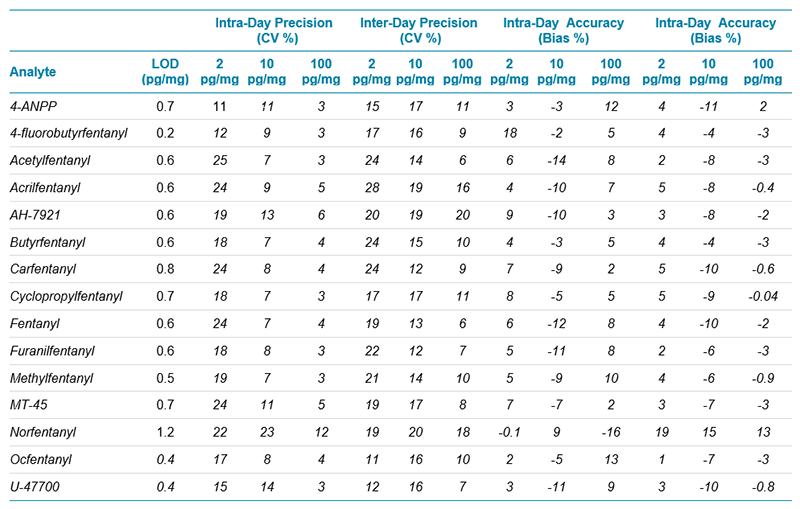 Click to enlarge
Click to enlarge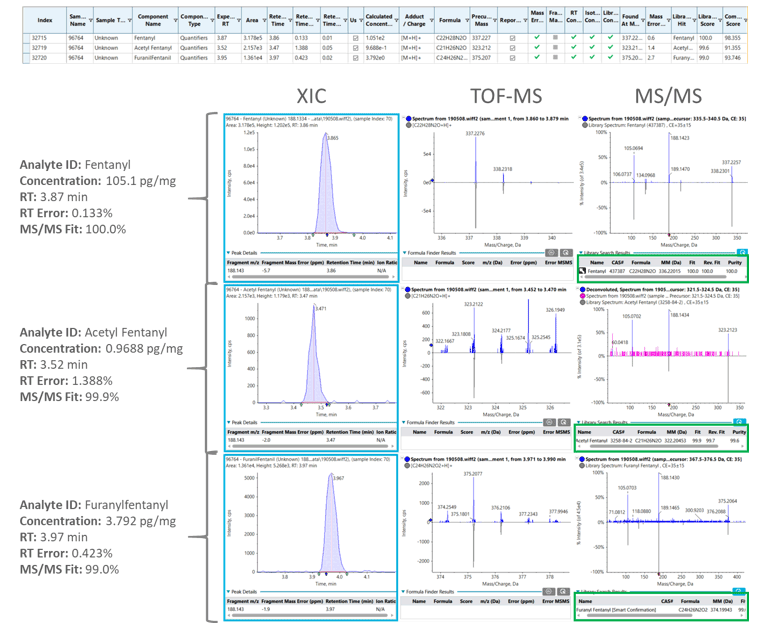 Click to enlarge
Click to enlarge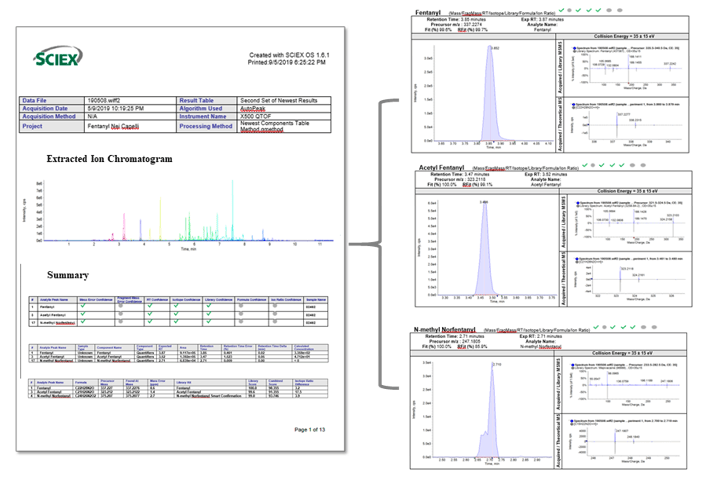 Click to enlarge
Click to enlarge