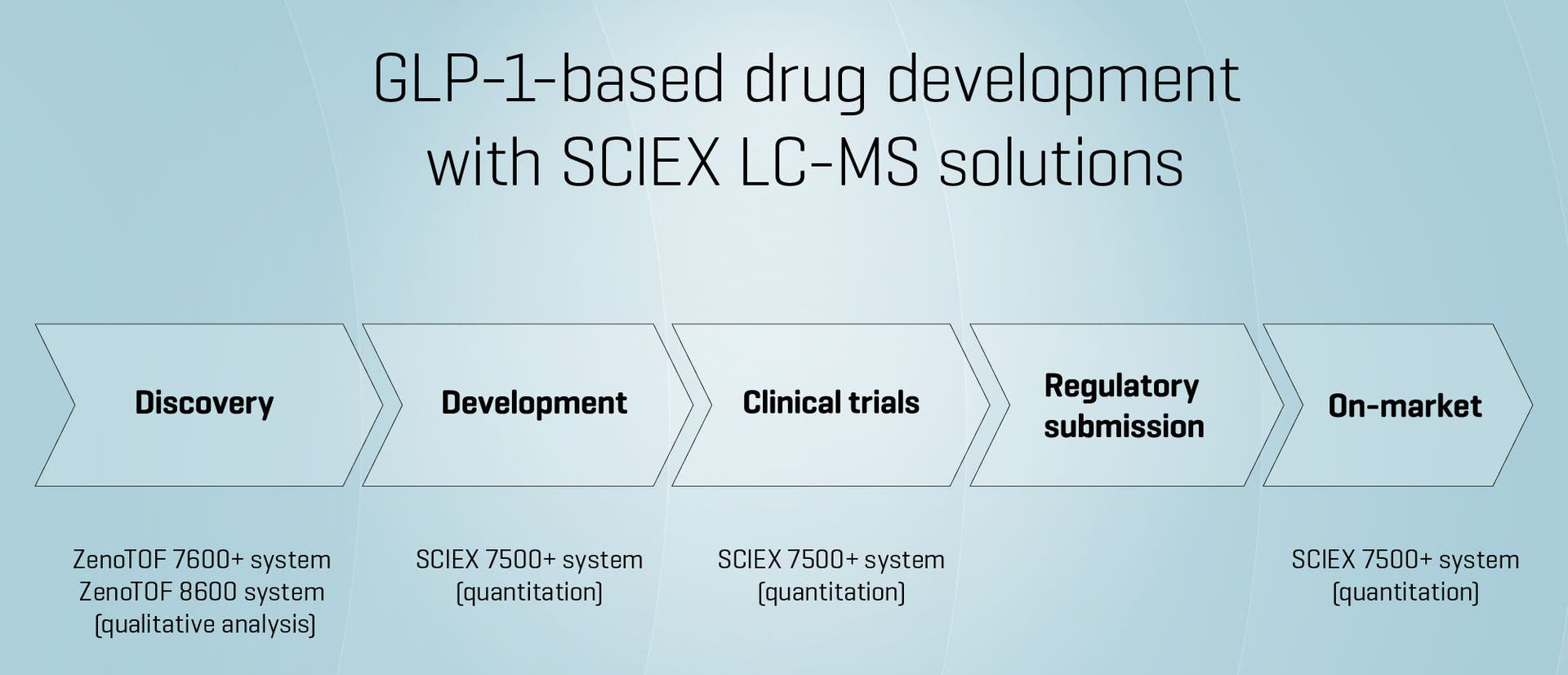
The development of GLP-1-based drugs, which are used to treat type 2 diabetes and obesity, requires precise, high-sensitivity analytical techniques to navigate the complexity of peptide therapeutics. While GLP-1 (glucagon-like peptide-1) is a well-characterized incretin hormone, its therapeutic analogs present unique challenges in drug discovery and development. These include low endogenous concentrations, structural complexity, susceptibility to degradation, and the need for detailed pharmacokinetic and metabolic profiling.
Liquid chromatography–mass spectrometry (LC-MS) has become an essential technique in this space, offering the sensitivity and specificity required to support the full lifecycle of GLP-1-based drug development, from early discovery through clinical validation.
Mass spectrometry in the development of GLP-1-based drugs
Advancing GLP-1 therapeutics with analytical innovation
Analytical challenges in GLP-1 drug development
- Sensitivity: GLP-1 and its analogs are present at low concentrations in biological matrices and are rapidly cleared, requiring highly sensitive detection methods.
- Structural complexity: These peptides have intricate three-dimensional structures, making it difficult to characterize biological activity, post-translational modifications, and degradation pathways.
- Susceptibility to modifications: GLP-1 analogs are prone to chemical and enzymatic degradation. Structural characterization is critical to ensure therapeutic integrity and efficacy.
- Sample preparation: Compared to small molecules, peptide drugs require more complex sample preparation workflows. Techniques often include protein precipitation, solid-phase extraction (SPE), and immunocapture. Challenges such as solubility limitations and non-specific binding must be carefully managed.
- MS method development: Poor ionization efficiency and fragmentation can hinder accurate quantitation and structural elucidation, necessitating optimized MS methods tailored to peptide behavior.
Quantitative analysis with the SCIEX 7500+ system
Workflow
The SCIEX 7500+ system delivers ultra-sensitive quantitation of GLP-1 analogs in complex biological matrices. Enhanced ion optics enable reliable detection of low-abundance peptides, supporting robust pharmacokinetic and bioavailability studies.
A new standard for instrument resilience and robustness. Engineered to maintain our highest sensitivity for up to twice as long in complex matrices.
Qualitative insights with the ZenoTOF 7600+ system and the ZenoTOF 8600 system
Workflow
For structural characterization, metabolite identification (MetID), and degradation analysis, the ZenoTOF 7600+ system and the ZenoTOF 8600 system provide high-resolution, accurate-mass data. These platforms are particularly effective for peptide characterization and identifying and localizing critical modifications.
Solution
Qualitative insights
Key innovations include:
- Electron activated dissociation (EAD): A powerful fragmentation technique that preserves labile modifications such as glucuronidation for confident drug metabolite identification in addition to providing unique fragments for comprehensive structural characterization.
- Zeno trap: Enhances sensitivity for low-level metabolites and degradants, enabling deeper insights into peptide stability and metabolism.
The SCIEX ZenoTOF 7600+ system is a Zeno trap-enabled QTOF engineered with the added specificity of the scanning quadrupole dimension to enhance speed, depth and certainty in quantitative measurements.
The newest accurate mass platform with sensitivity levels up to 10x greater than existing SCIEX technology. This translates not only into the lower limits of quantitation but also into new enabled approaches to making biologically relevant discoveries that can be proved by the data.
Related resources
-
Hub
Quantitative bioanalysis
Unlock reliable and sensitive quantitative biological data with LC-MS solutions that work as hard as you do
-
Technical note
Quantitative performance of a next-generation, highly robust triple quadrupole mass spectrometer
Bioanalytical laboratories are constantly challenged by the need for reliable triple quadrupole mass spectrometers to ensure the delivery of proper quantitative performance. In order to effectively meet the required drug discovery and development timelines, bioanalytical workflows need robust mass spectrometers to minimize instrument downtime.
-
Technical note
Low-ng/mL quantitation of glucagon-like peptide-1 (GLP-1) analog in rat plasma
This technical note demonstrates a sensitive method to quantify a glucagon-like peptide-1 (GLP-1) analog, semaglutide, in rat plasma on a high-end triple quadrupole mass spectrometer. A lower limit of quantitation (LLOQ) of 0.2 ng/mL was determined using a 10-minute LC-MS/MS method (Figure 1).
-
Technical note
Improved LC-MRM workflow for quantification of glucagon like peptide-1 analogues
In this technical note, a robust and sensitive LC-MRM method was developed for the quantification of liraglutide, a clinically approved glucagon-like peptide-1 (GLP-1) analogue.



