Ultrasensitive bioanalytical quantification with minimal method development time
Routinely achieve low-level quantification with the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Rolf Kern, Christie Hunter
SCIEX, USA
Introduction
As the pharmaceutical industry continues to explore new, less conventional chemical classes for highly potent therapeutics, the bioanalytical scientist must continue to support early stage in vivo work by generating high-quality data in increasingly shorter times. Meeting the sensitivity requirements for new and difficult compounds dosed at very low levels can be challenging, and often requires significant sample preparation (i.e. solid phase or liquid-liquid extraction), complex chromatography (2 dimensional or trap & elute LC), or a combination of both. The time to actually perform these processes can be streamlined through automation, but development of an appropriate method can be time consuming, especially considering the timelines most bioanalytical departments operate under. With the introduction of the SCIEX 7500 System, the improved sensitivity over the already industry established QTRAP® 6500+ LC-MS/MS System will enable bioanalytical groups to generate sensitive, relevant data, without the need for complicated, specific sample preparation and complex chromatography.
Here, the utility of the highly sensitive SCIEX 7500 System for the analysis of monomethyl auristatin E (MMAE, a highly cytotoxic compound requiring very low detection limits) in rat plasma was demonstrated. A very simple protein precipitation was used for sample preparation followed by a generic, direct-injection method using microflow chromatography for even higher sensitivity. This enabled detection limits of 2.5 pg/mL in rat plasma to be achieved.
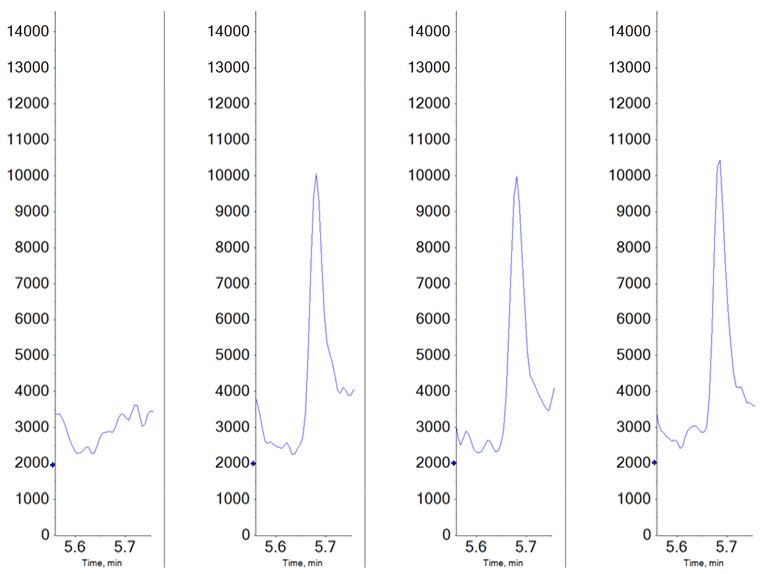
Figure 1. Data quality at lower limit of quantification for MMAE. Data for the MMAE MRM transition is shown here for the blank (left) as well as three replicate injections of the 2.5 pg/mL sample.
Key feature of SCIEX 7500 System with microflow LC
- The SCIEX 7500 System provides high sensitivity for bioanalytical quantification, with improvements in ion generation, due to the OptiFlow® Pro Ion Source with E Lens™ Technology, and in ion sampling, due to the D Jet™ Ion Guide1
- Ease of coupling microflow chromatography with the OptiFlow Pro Ion Source provides increased sensitivity with similar robustness and speed of analysis to analytical flow LC2
- Simplified sample preparation for streamlined method development was possible due to the increased sensitivity of the MS and LC approaches
- Powered by SCIEX OS Software, an easy to learn, easy to use, all-in-one platform for acquisition through data reporting
- The combination of the SCIEX 7500 System with microflow chromatography enables detection limits down to 2.5 pg/mL in rat plasma

 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge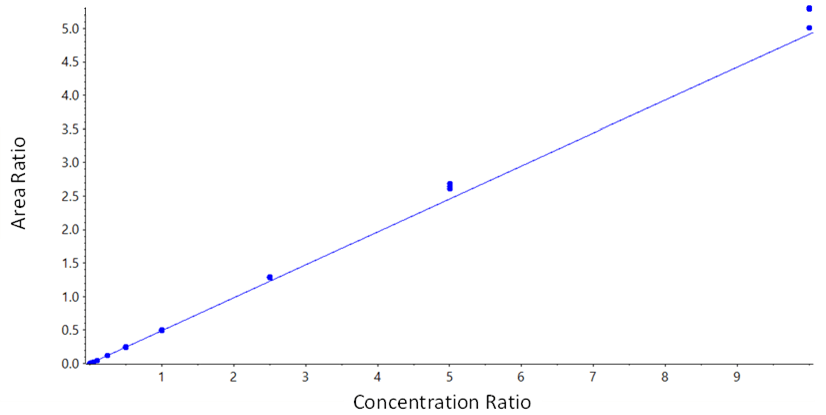 Click to enlarge
Click to enlarge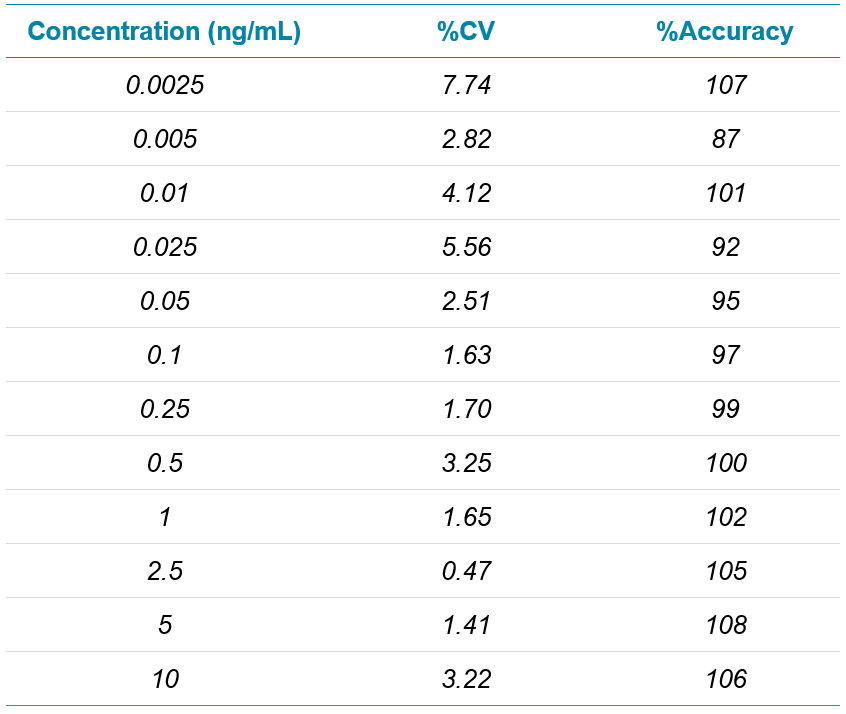 Click to enlarge
Click to enlarge