Ultrasensitive bioanalytical quantification with minimal method development time
Routinely achieve low-level quantification with the SCIEX Triple Quad™ 7500 LC-MS/MS System – QTRAP® Ready
Rolf Kern, Christie Hunter
SCIEX, USA
Introduction
As the pharmaceutical industry continues to explore new, less conventional chemical classes for highly potent therapeutics, the bioanalytical scientist must continue to support early stage in vivo work by generating high-quality data in increasingly shorter times. Meeting the sensitivity requirements for new and difficult compounds dosed at very low levels can be challenging, and often requires significant sample preparation (i.e. solid phase or liquid-liquid extraction), complex chromatography (2 dimensional or trap & elute LC), or a combination of both. The time to actually perform these processes can be streamlined through automation, but development of an appropriate method can be time consuming, especially considering the timelines most bioanalytical departments operate under. With the introduction of the SCIEX 7500 System, the improved sensitivity over the already industry established QTRAP® 6500+ LC-MS/MS System will enable bioanalytical groups to generate sensitive, relevant data, without the need for complicated, specific sample preparation and complex chromatography.
Here, the utility of the highly sensitive SCIEX 7500 System for the analysis of monomethyl auristatin E (MMAE, a highly cytotoxic compound requiring very low detection limits) in rat plasma was demonstrated. A very simple protein precipitation was used for sample preparation followed by a generic, direct-injection method using microflow chromatography for even higher sensitivity. This enabled detection limits of 2.5 pg/mL in rat plasma to be achieved.
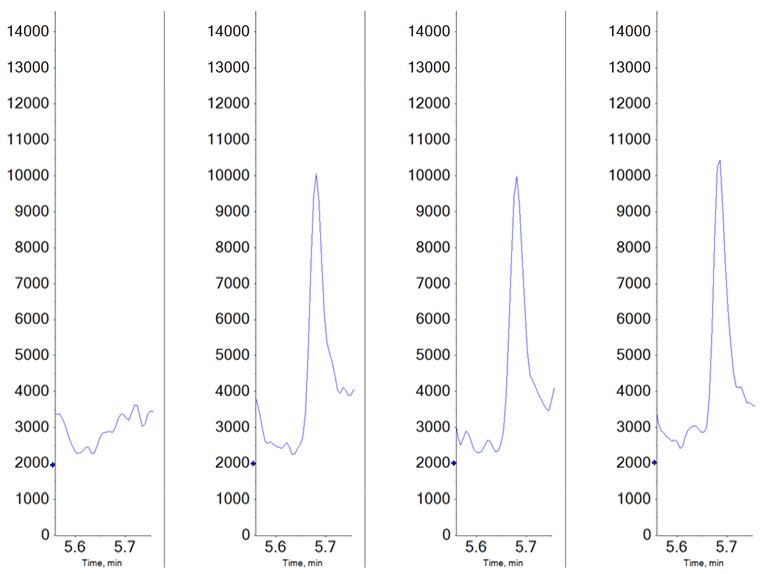
Figure 1. Data quality at lower limit of quantification for MMAE. Data for the MMAE MRM transition is shown here for the blank (left) as well as three replicate injections of the 2.5 pg/mL sample.
Key feature of SCIEX 7500 System with microflow LC
- The SCIEX 7500 System provides high sensitivity for bioanalytical quantification, with improvements in ion generation, due to the OptiFlow® Pro Ion Source with E Lens™ Technology, and in ion sampling, due to the D Jet™ Ion Guide1
- Ease of coupling microflow chromatography with the OptiFlow Pro Ion Source provides increased sensitivity with similar robustness and speed of analysis to analytical flow LC2
- Simplified sample preparation for streamlined method development was possible due to the increased sensitivity of the MS and LC approaches
- Powered by SCIEX OS Software, an easy to learn, easy to use, all-in-one platform for acquisition through data reporting
- The combination of the SCIEX 7500 System with microflow chromatography enables detection limits down to 2.5 pg/mL in rat plasma
Methods
Sample preparation: Monomethyl auristatin E (MMAE) was obtained from Achemblock (cat# 10353), and the internal standard, dolastatin-15 was obtained from Sigma (product # D5566). Stock solutions were prepared to 1 mg/mL for each in DMSO and stored at -20 ºC until use. Working standards were prepared by serial dilution to 10x the final concentrations. Standards were prepared by adding 10 µL of working standard to 100 µL of K2EDTA rat plasma (Bioreclamation IVT).
Standards and blanks were prepared for analysis by adding 300 µL of acetonitrile, containing 10 ng/mL of the internal standard, to 100 µL of sample to precipitate plasma proteins. The samples were vortexed for 1 min, then centrifuged at 14k rpm for 15 min. The supernatant was transferred to a clean Eppendorf tube and evaporated to near dryness under N2. The samples were reconstituted with 100 µL 5% methanol in water and transferred to insert lined HPLC vials for analysis.
Chromatography: The nanoLC™ 425 System (SCIEX) with a 1-10 µL/min flow module and a 10 µL injection loop, operated in direct injection mode was used for separation. Mobile phase A was water with 0.1% formic acid by volume and mobile phase B was acetonitrile with 0.1% formic acid by volume. The column was a Phenomenex 0.3 x 50 mm Luna C18 (Phenomenex part # 00B-4251-AC). A simple 5-95% B gradient was used at a flow rate of 5 mL/min, with a total run time of 9 minutes which included the sample load time and column re-equilibration.
Mass spectrometry: A SCIEX 7500 System, operated in positive ESI MRM mode was used for data acquisition. The MRM transitions monitored are listed in Table 1, and the source conditions are listed in Table 2.
Table 1. MRM parameters.
Table 2. Source conditions.
Data processing: MS data acquisition was performed using SCIEX OS Software. Data processing was performed using the Analytics module in SCIEX OS Software. The calibration curve was run in triplicate with good linearity from 2.5 pg/mL to 10 ng/mL (Figure 2) with good accuracy and precision for all concentrations, shown in Table 3.
Figure 2. Calibration curve for MMAE in rat plasma. Very good linearity was observed for MMAE in rat plasma measured over a concentration range for 2.5 pg/mL to 10 ng/mL.
Table 3. Summary statistics from the calibration curve. Reproducibility and accuracy results from the calibration curve shows excellent %CV and %Accuracy for 3 replicates.
Requirement for very high sensitivity detection
Monomethyl auristatin E (MMAE) is a highly cytotoxic compound that is a synthetic analogue of the naturally occurring dolastatin class of compounds. It works by inhibiting the formation of tubulin during cellular division, and while it is far too toxic to be used independently as a cancer therapeutic, it is used as the toxic component of some antibody drug conjugates (Adcetris, Padcev). Monitoring of the unconjugated toxin is required as part of ADC clinical trials to ensure the drug linker, which binds the toxic payload to the antibody, does not release the toxic compound prior to entering the targeted cancer cells. Levels of unconjugated toxin are expected to be very low, and because they are so acutely toxic in their free state, very low detection limits are required to accurately and relevantly monitor for their presence.
Combining the well characterized sensitivity improvements of microflow HPLC with its ability to improve ionization efficiency, along with a highly sensitive triple quadrupole mass spectrometer, provided a very effective platform for routine, highly sensitive bioanalytical assays. Here, complex sample preparation and chromatography development time was minimized, so less time was spent on development, allowing results to be generated more quickly.
Figure 2. Calibration curve for MMAE in rat plasma. Very good linearity was observed for MMAE in rat plasma measured over a concentration range for 2.5 pg/mL to 10 ng/mL.
Table 3. Summary statistics from the calibration curve. Reproducibility and accuracy results from the calibration curve shows excellent %CV and %Accuracy for 3 replicates.
Conclusions
The method and data presented here illustrate the utility of using the SCIEX 7500 System in conjunction with microflow chromatography as an analytical platform for routine, highly sensitive bioanalytical sample analysis. The 2.5 pg/mL LLOQ that was observed for MMAE in rat plasma was achieved using a generic, unoptimized protein precipitation sample preparation, and a generic HPLC gradient with standard stationary phase and mobile phases. The ability to routinely detect ultra-low analyte levels, with minimal method development time, will allow bioanalytical groups to deliver high quality data with rapid turnaround times.
References
- Enabling new levels of quantification. SCIEX technical note RUO-MKT-02-11886-A.
- Microflow chromatography: Bridging the sensitivity gap for quantitation of a pyrrolobenzodiazepine dimer. SCIEX technical note RUO-MKT-02-9512-A.
- Pharmacologically relevant measurement of high potency drug candidates with improved sensitivity in in-vivo transporter assays. SCIEX technical note RUO-MKT-02-11960-A.

 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge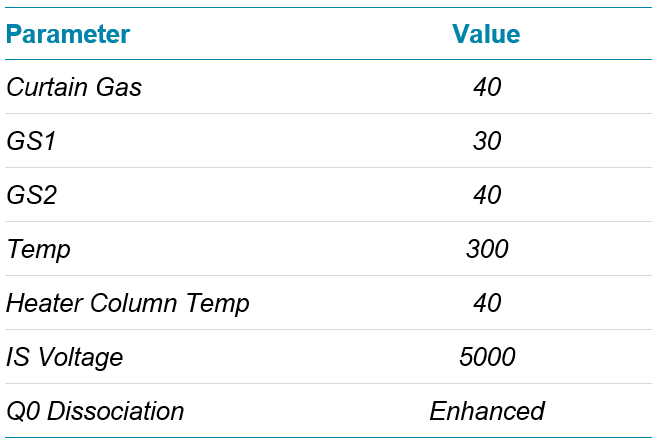 Click to enlarge
Click to enlarge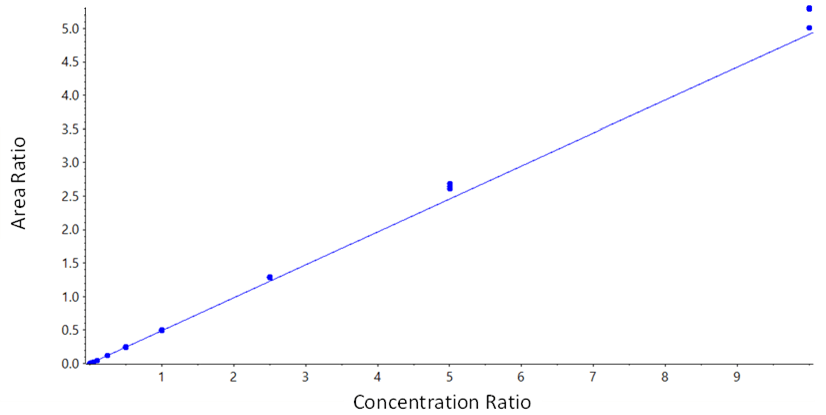 Click to enlarge
Click to enlarge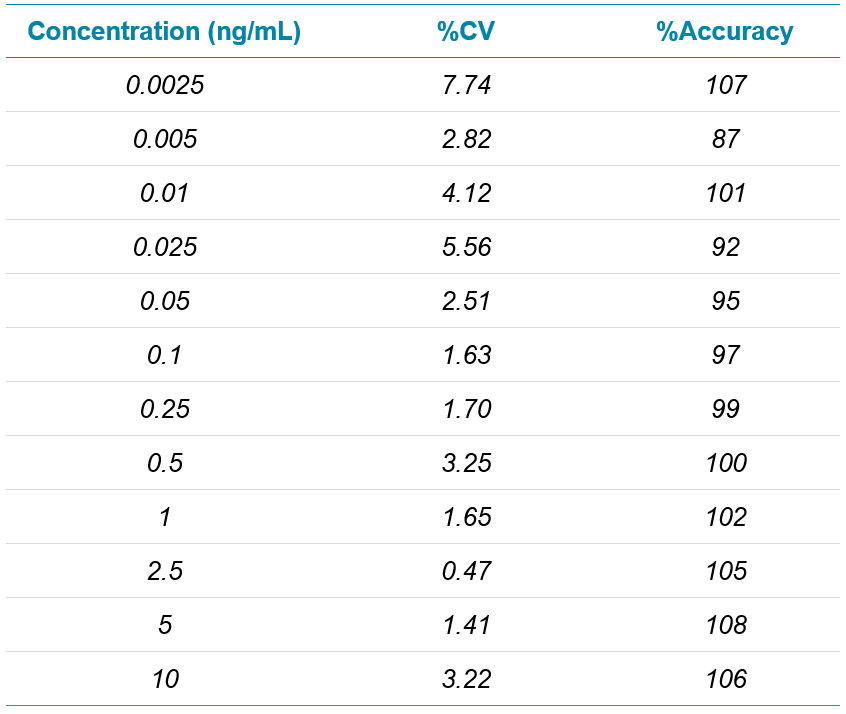 Click to enlarge
Click to enlarge