Analysis of genotoxic nitrosamines in losartan using the SCIEX 7500 system
Exceptional quantitative sensitivity for confident analysis of contaminants
Dilip Kumar Reddy Kandula, Santosh Kapil Kumar Gorti, Anthony Romanelli
SCIEX, USA
Abstract
Since the discovery of genotoxic nistrosamines in pharmaceutical products in 2018, sensitive and selective methods for quantification of these contaminants has become critical. The method presented here provides good chromatographic separation of the six analyzed nitrosamine compounds from the losartan API. Good linearity over the concentration range monitored was observed and the lower limits of quantification for each compound translate to daily intake limits significantly lower than those currently specified by the FDA and EMA.

Introduction
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) continue to closely monitor nitrosamine levels in pharmaceutical products since the initial discovery of N-nitrosodimethylamine (NDMA) in valsartan in 2018 prompted recalls of the drug. Later in 2018, N-nitrosodiethylamine (NDEA) was also found in some lots of valsartan, prompting further recalls. In 2019, several lots of losartan were recalled1 due to the presence of another nitrosamine contaminant, N-nitroso-N-methyl-4-amino butyric acid (NMBA). This was the first recall of an angiotensin receptor blocker (ARB) medicine due to NMBA contamination. These findings and recalls highlight the ongoing need for sensitive, selective methods for the analysis of multiple nitrosamines in pharmaceutical products prone to contamination by these potentially carcinogenic compounds.
The method presented here shows the utility of the highly sensitive SCIEX 7500 system for the analysis of six commonly monitored nitrosamines in losartan. Atmospheric pressure chemical ionization (APCI) is the preferred mode of ionization for nitrosamine assays, but a sensitivity comparison with the much more broadly used electrospray ionization (ESI) technique was performed to explore the sensitivity difference between these approaches. Lower limits of quantification (LLOQ’s) of between 0.01 ng/mL to 0.10 ng/mL, depending on the analyte, were reported for the nitrosamine compounds in this study.
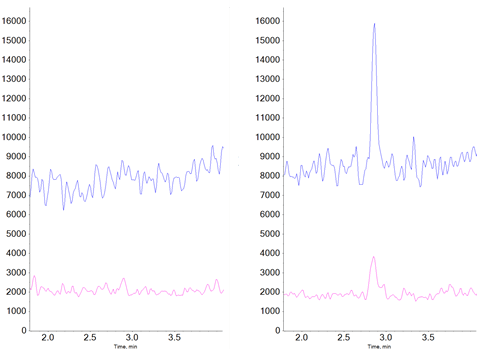
Figure 1. Detection of NDMA. Blank (left) and 0.01 ng/ml LLOQ (right) for NDMA is shown. Quantitative MRM transitions are shown in blue, confirmation MRMs are shown in pink.
Key features of the SCIEX 7500 system for nitrosamine monitoring
- E Lens probe and the D Jet ion guide 2 provide enhanced sensitivity through significant gains in the generation, capture and transmission of ions
- The OptiFlow Pro ion source provides flexibility and ease of use, with easily interchangeable ESI and APCI towers.
- SCIEX OS software is an easy-to-learn, easy-to-use platform for data acquisition and processing that is fully compliant with 21 CFR Part 11
 Click to enlarge
Click to enlarge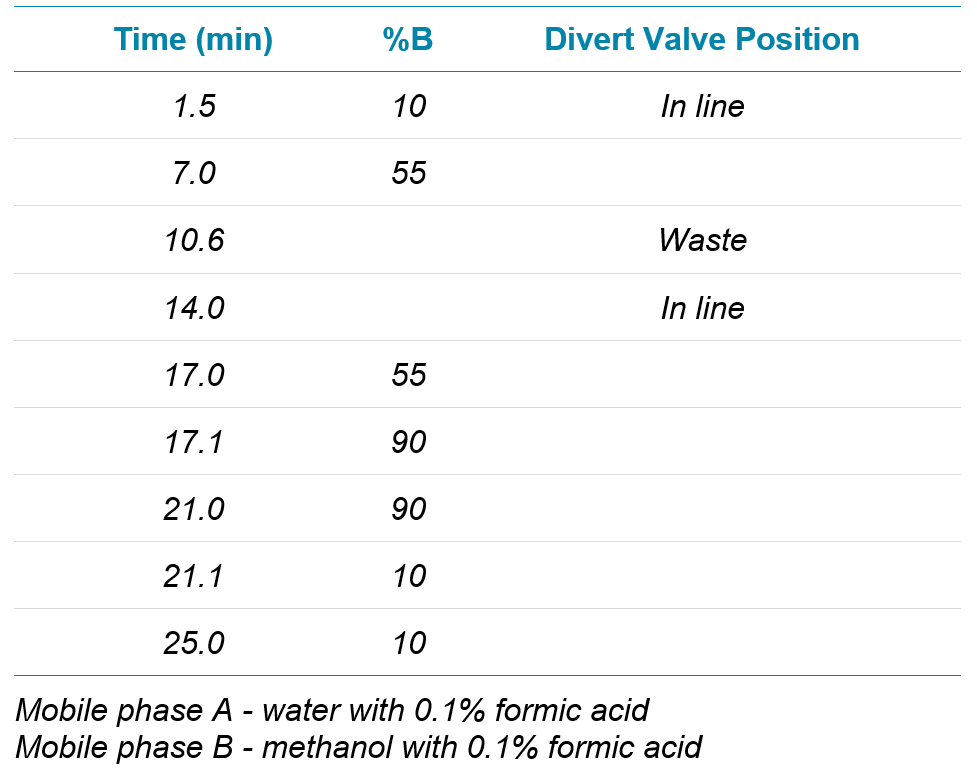 Click to enlarge
Click to enlarge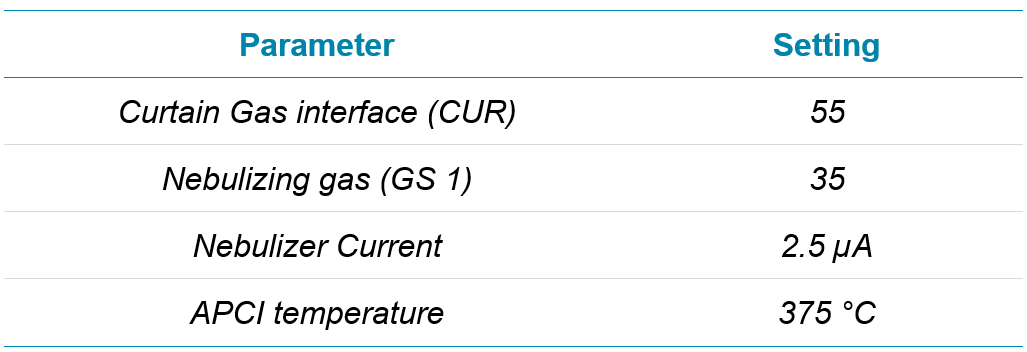 Click to enlarge
Click to enlarge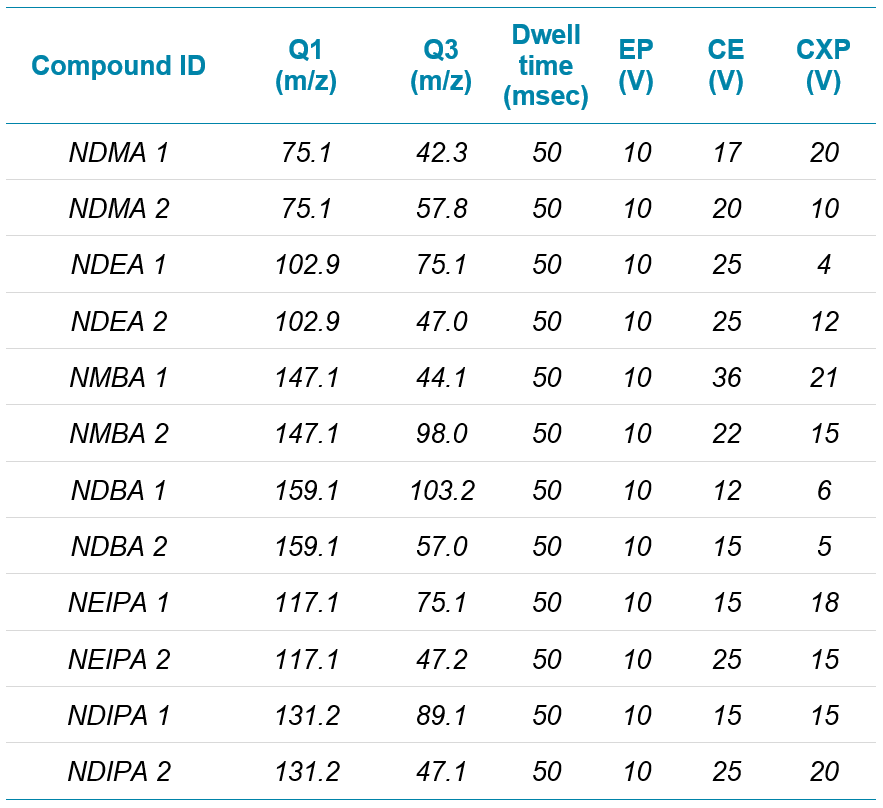 Click to enlarge
Click to enlarge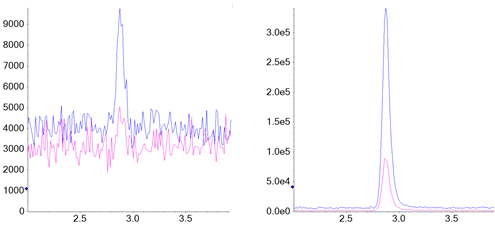 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge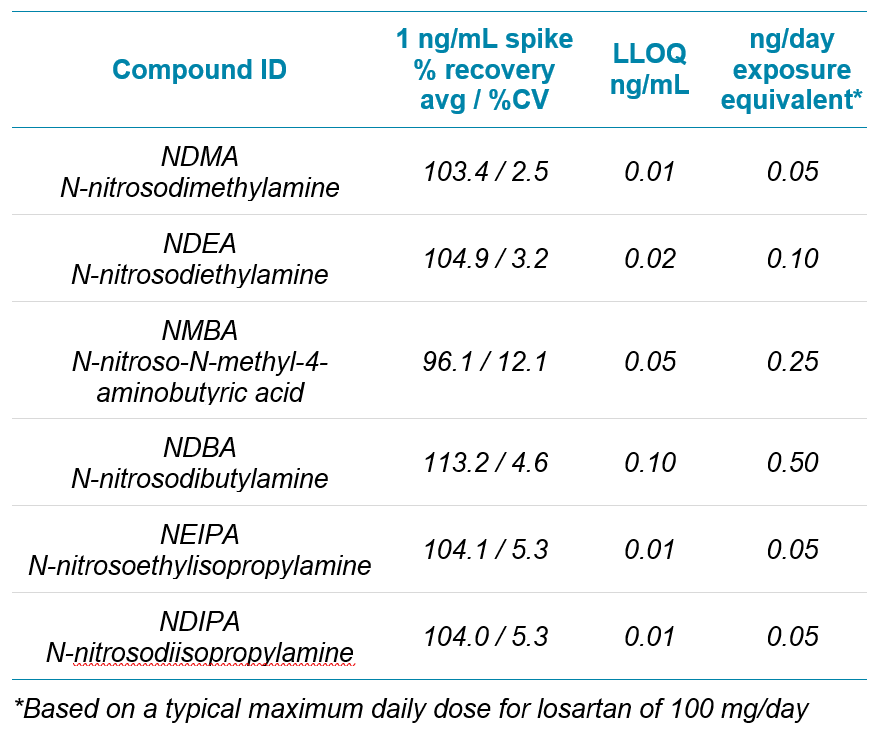 Click to enlarge
Click to enlarge Click to enlarge
Click to enlarge