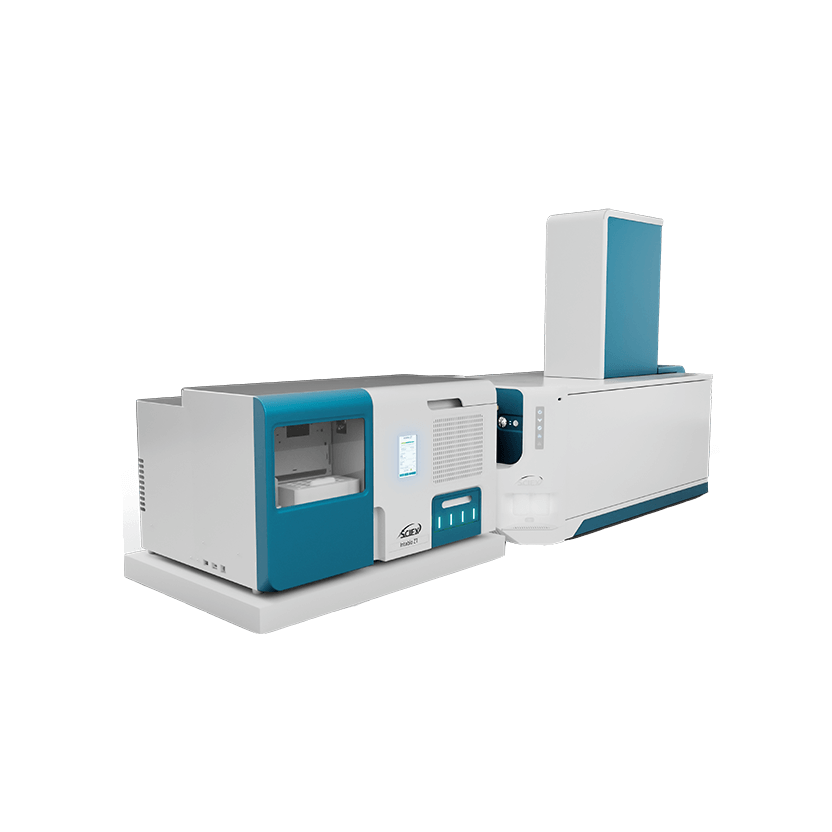
The Intabio ZT system puts the critical information needed to make informed decisions on developability in the hands of biopharma scientists throughout the drug development pipeline. It cuts out information gaps and reduces risk to move biotherapeutic development forward faster.
Discover how your peers in the biopharma world are rewriting the rules of charge variant analysis, incorporating icIEF-UV/MS workflows on the Intabio ZT system into projects that are both challenging and routine.