There are various MS/MS scan types for qualitative studies, but the most widely used is the product ion scan. This involves selecting precursor ions based on their mass, fragmenting them, and analyzing the resulting product ions. This process uses two stages of mass filtering with a fragmentation event in between. The masses and mass differences of the product ions help determine the structure or sequence of the original molecule, and the energy required for fragmentation reveals the nature of specific chemical bonds.
For MS/MS to be effective, fragmentation must be interpretable and reproducible. The energy transferred must be "just right"—sufficient to produce diagnostic product ions without obliterating the parent molecule. Even with the right energy, fragmentation can be insufficient, leaving gaps in the spectrum or missing key diagnostic ions. Important side chains and modifications might be cleaved off, leaving no indication of their location.
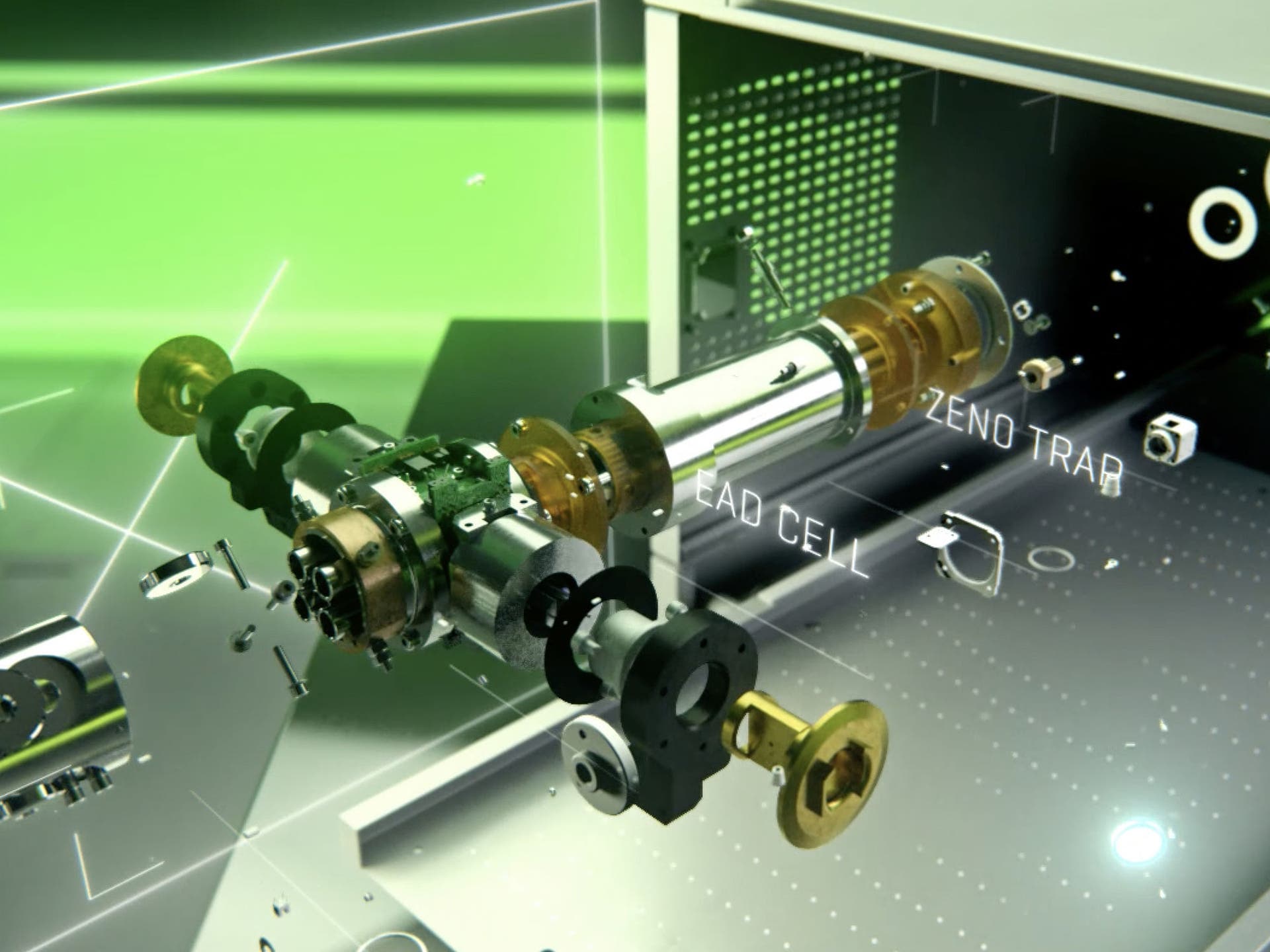
Various mechanisms have been used for fragmenting ions, including photons, electrons, atoms, molecules, and solid surfaces. The most common techniques use atoms, molecules, or electrons to impart energy for fragmentation.







.jpg?quality=75&width=1920)