La solución más completa para análisis cuantitativos de muestras biológicas
La solución de cuantificación de muestras biológicas de SCIEX le ofrece todo lo que necesita para pasar al bioanálisis de moléculas grandes. Con la plataforma de cuantificación de muestras biológicas probada en laboratorio más completa del mercado, podrá simplificar el desarrollo de métodos, acelerar sus flujos de trabajo y obtener resultados exactos más rápido que nunca.
La automatización integrada supone un importante ahorro de tiempo
La solución de cuantificación de muestras biológicas de SCIEX no solo proporciona protocolos probados previamente, sino que también ofrece métodos de preparación automatizada de muestras con la estación de trabajo de automatización de laboratorio Beckman Coulter Biomek. El resultado: mayor productividad con resultados más coherentes y fiables.
Flexible
Integre todos los aspectos de la manipulación de líquidos (pipeteado, calentamiento y mezcla) en un único sistema automatizado tan potente como flexible y fácil de usar.
Intuitivo
No necesita ser un experto en automatización. El software iniciador de métodos de SCIEX guía a los operadores a través del flujo de trabajo de cuantificación de muestras biológicas preintegrado y esto los mantiene actualizados sobre los avances en el proceso y les permite supervisar el estado de forma remota.
Capacidad
En combinación con las aplicaciones de vMethod™ probadas en laboratorio, podrá procesar grandes lotes de muestras con poco esfuerzo y una fiabilidad superior.
Impulse sus flujos de trabajo
Los flujos de trabajo de la solución Biologics Quant incorporan herramientas avanzadas probadas en laboratorio como vMethod™, DiscoveryQuant™ y MultiQuant™ para ayudarle a poner en marcha rápidamente sus estudios de moléculas grandes y acelerar significativamente el tiempo de desarrollo del método.

Productivo
Las aplicaciones de vMethod le permiten seleccionar entre diversos métodos preintegrados probados y asignarlos a los compuestos de interés, lo que garantiza resultados coherentes y portátiles.

Optimizado
El software DiscoveryQuant optimiza los métodos y gestiona el análisis simultáneo de cientos de moléculas pequeñas o grandes con un proceso rápido, potente y fiable.
Seguro
El software MultiQuant le permite gestionar grandes conjuntos de datos para elaborar de forma eficaz informes bioanalíticos de todos los tipos de moléculas e incluye funciones de cumplimiento mejoradas.
Cuantificación de absoluta confianza
La solución Biologics Quant está específicamente diseñada para afrontar cualquier reto de cuantificación biológica. Combina el mejor hardware de su clase con una sensibilidad extremadamente alta y un rango dinámico lineal para la cuantificación de productos bioterapéuticos en matrices de muestras preclínicas o clínicas.

Confianza total
El potente sistema de LC-MS QTRAP® 6500+ de alta sensibilidad está probado en las condiciones analíticas más exigentes.

Estabilidad excepcional
El sistema SCIEX ExionLC™ ofrece una estabilidad basal excepcional, mientras que el sistema SCIEX M3 MicroLC ofrece una alta sensibilidad para cantidades de muestra limitadas.
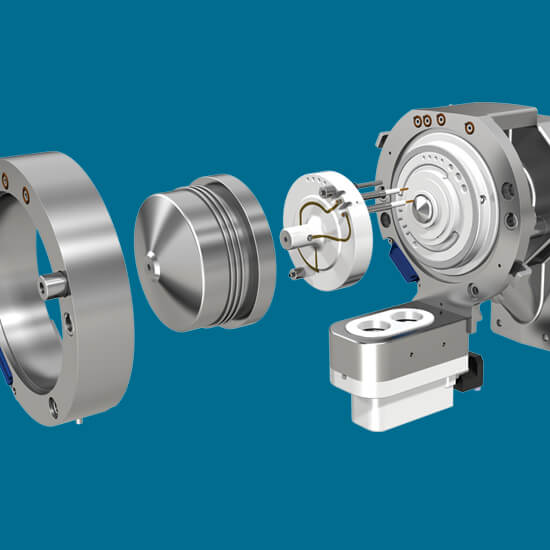
Selectividad mejorada
Gestione las interferencias de compuestos complejos mejorando la selectividad con la tecnología de movilidad diferencial SelexION®.
Cuantificación probada para todos sus productos bioterapéuticos
Tanto si va a analizar péptidos terapéuticos, anticuerpos monoclonales o los conjugados fármaco-anticuerpo más complejos, esta solución proporciona datos de alta calidad para la cuantificación de una amplia gama de productos bioterapéuticos en matrices de muestras clínicas y preclínicas.
Péptidos terapéuticos
Análisis sensibles y fiables de péptidos terapéuticos, como el glucagón y la insulina glargina
Anticuerpos monoclonales
Detección selectiva de anticuerpos monoclonales bioterapéuticos
Conjugados fármaco-anticuerpo
Cuantificación de alta calidad de conjugados fármaco-anticuerpo (CFA)
Todo lo que necesita para el bioanálisis de macro moléculas
SCIEX ofrece configuraciones flexibles para ayudarle a conseguir sus objetivos de cuantificación biológica. Podemos ofrecerle métodos de preparación automatizada de muestras, un hardware de LC-MS de primera clase, un software de procesamiento de datos, completos métodos preintegrados y soluciones de formación para acelerar el paso a la cuantificación de macro moléculas grandes y llevar su laboratorio de bioanálisis al siguiente nivel.
Estación de trabajo de automatización Biomek
Estación de trabajo de automatización fácil de usar para proporcionar una mayor productividad y resultados fiables.
Sistema QTRAP 6500+ con aplicaciones Pharma Biologics vMethod™
Consiga el alto nivel de sensibilidad y selectividad, y el rango dinámico necesarios para procedimientos de cuantificación complejos.
Paquetes de software
Aumente la productividad con el software DiscoveryQuant™ y el software MultiQuant™.
Pharma Biologics vMethods
Aplicaciones de vMethod probadas en laboratorio para garantizar resultados uniformes y portabilidad entre laboratorios.